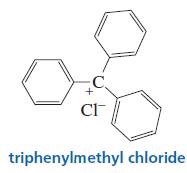
The triphenylmethyl cation is so stable that a salt such as triphenylmethyl chloride can be isolated and
Question:
The triphenylmethyl cation is so stable that a salt such as triphenylmethyl chloride can be isolated and stored. Why is this carbocation so stable?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:




