(a) For each of the two reactions shown in Fig. P17.58, suggest a mechanism that is consistent...
Question:
(a) For each of the two reactions shown in Fig. P17.58, suggest a mechanism that is consistent with all of the experimental facts given.
Experimental observations:
(1) Both reactions conform to the following rate law, although the rate constants for each reaction are different.![]()
(2) The alkyl chloride starting materials do not interconvert under the reaction conditions.
(3) The following compound, prepared separately, is not converted into the observed product under the reaction conditions.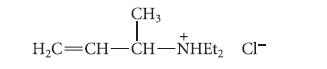
In particular, explain the importance of facts (2) and (3) in understanding the mechanism.
(b) The mechanism of reaction 2 is called the SN2′ mechanism. Suggest a reason why this reaction occurs by the SN2′ mechanism and reaction 1 does not.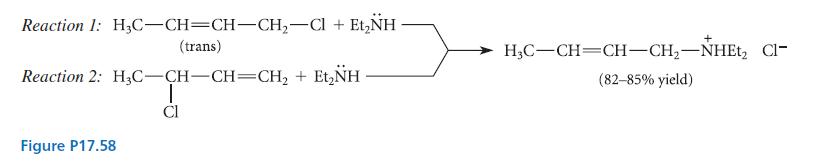
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





