In the late 1970s, a graduate student at a major West Coast university began synthesizing new classes
Question:
In the late 1970s, a graduate student at a major West Coast university began synthesizing new classes of drugs and testing them on himself. After being expelled from the university, he began making his living by illegally synthesizing and selling to heroin addicts compound B, a synthetic analog of meperidine (Demerol). (See Fig. P17.57) After shortening his synthetic procedure and self-injecting his product, he developed severe symptoms of Parkinson’s disease, as did several of his young clients; one person died. Chemists found that his compound B contained two by-products, alcohol C and another compound MPTP (C12H15N), which, when independently prepared and injected into animals, caused the same symptoms. (Ironically, this has been one of the most significant advances in Parkinsonism research.)
Given the illicit chemistry outlined in Fig. P17.57, provide the structure of compound A, suggest a structure for MPTP, and show how all products are formed.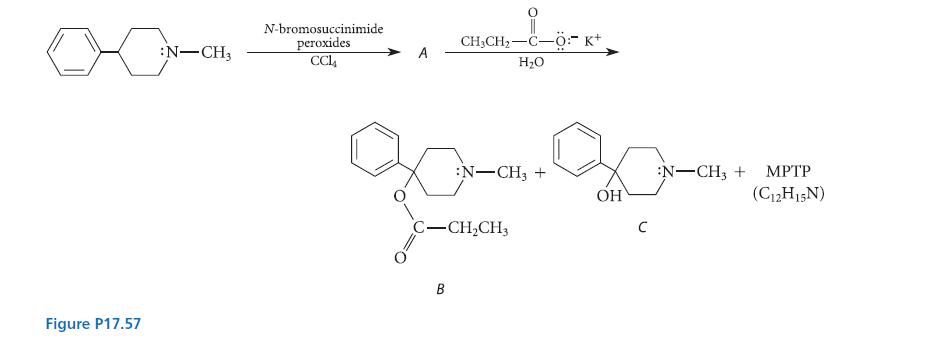
Step by Step Answer:






