At 100 C, d-idose exists mostly (about 86%) as a 1,6-anhydropyranose: (a) Draw the chair conformation of
Question:
At 100 °C, d-idose exists mostly (about 86%) as a 1,6-anhydropyranose: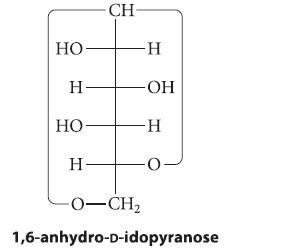
(a) Draw the chair conformation of this compound.
(b) Explain why d-idose has more of the anhydro form than d-glucose. (Under the same conditions, glucose contains only 0.2% of the 1,6-anhydro form.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





