Question: L-Ascorbic acid (vitamin C) has the following structure: (a) Ascorbic acid has pK a = 4.21, and is thus about as acidic as a typical
L-Ascorbic acid (vitamin C) has the following structure: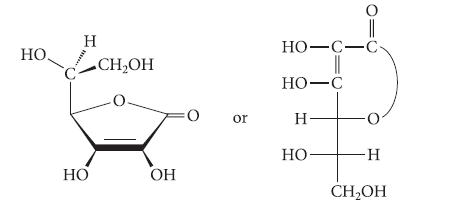
(a) Ascorbic acid has pKa = 4.21, and is thus about as acidic as a typical carboxylic acid. Identify the acidic hydrogen and explain.
(b) Thousands of tons annually of ascorbic acid are made commercially from d-glucose. In the synthesis shown in Fig. P24.60, give the structures of the compounds A–C.
. H HO CHOH -0- or 0=0 -- - H- CHOH
Step by Step Solution
3.49 Rating (149 Votes )
There are 3 Steps involved in it
a The acidic hydrogen is the one that ionizes to give a conjugatebase anion in which the negative ch... View full answer

Get step-by-step solutions from verified subject matter experts


