Consider the relative rates of the two solvolysis reactions in acetic acid solvent shown in Fig. P17.50.
Question:
Consider the relative rates of the two solvolysis reactions in acetic acid solvent shown in Fig. P17.50.
(a) Suggest a reason that compound A undergoes solvolysis much faster than compound B.
(b) Account for the retention of stereochemistry observed in reaction A with a mechanism.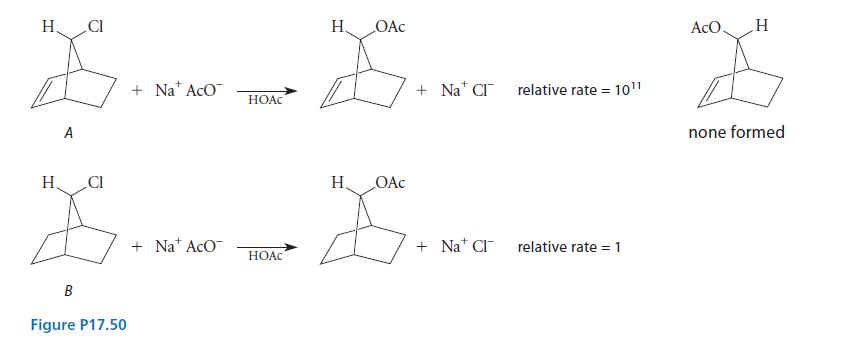
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





