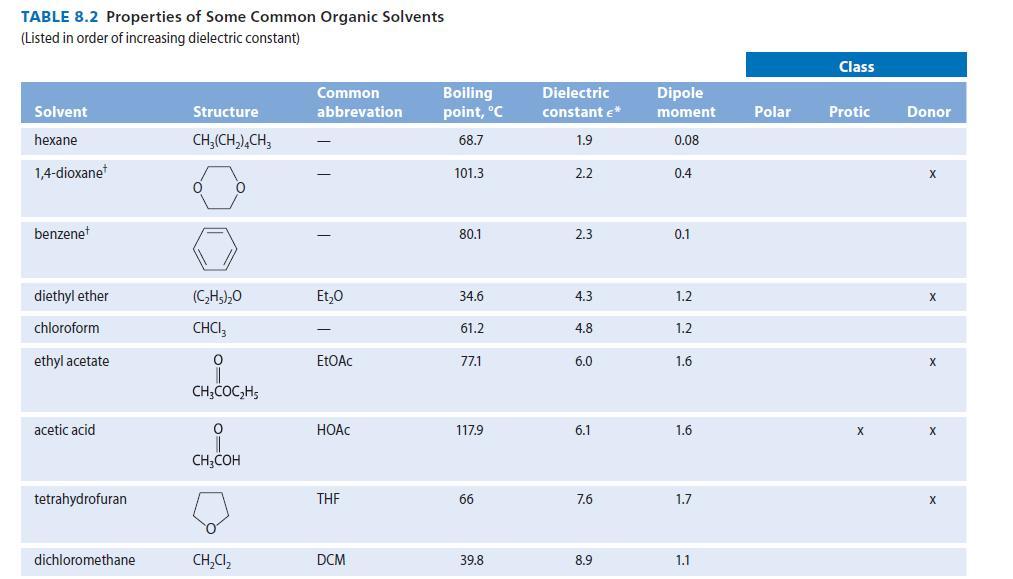
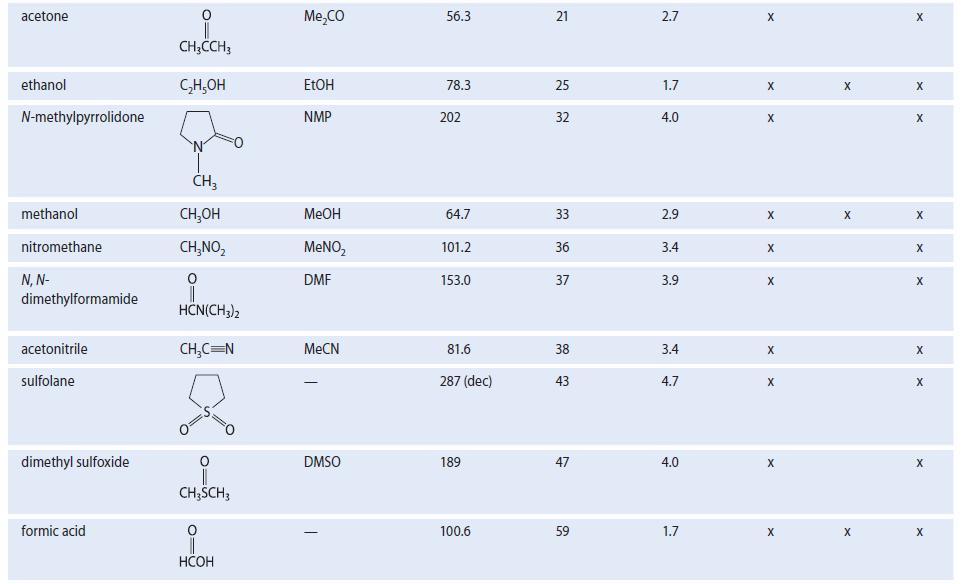
Dimethyl sulfoxide (DMSO, Table 8.2) has a very large dipole moment (4.0 D). Using structures, show the
Question:
Dimethyl sulfoxide (DMSO, Table 8.2) has a very large dipole moment (4.0 D). Using structures, show the stabilizing interactions to be expected between DMSO solvent molecules and
(a) A dissolved sodium ion;
(b) Dissolved water;
(c) A dissolved chloride ion.

Transcribed Image Text:
TABLE 8.2 Properties of Some Common Organic Solvents (Listed in order of increasing dielectric constant) Solvent hexane 1,4-dioxane benzenet diethyl ether chloroform ethyl acetate acetic acid tetrahydrofuran dichloromethane Structure CH₂(CH₂), CH3 0 (C₂H5)₂0 CHCI 0 CH3COC₂H5 0 CH3COH CH₂Cl₂ Common abbrevation Et₂0 EtOAc HOAC THE DCM Boiling point, °C 68.7 101.3 80.1 34.6 61.2 77.1 117.9 66 39.8 Dielectric constant €* 1.9 2.2 2.3 4.3 4.8 6.0 6.1 7.6 8.9 Dipole moment 0.08 0.4 0.1 1.2 1.2 1.6 1.6 1.7 1.1 Polar Class Protic X Donor X X X X X
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
1 a Below are represented donor interactions DMSO molecules function as Lewi...View the full answer

Answered By

Felix Onchweri
I have enough knowledge to handle different assignments and projects in the computing world. Besides, I can handle essays in different fields such as business and history. I can also handle both short and long research issues as per the requirements of the client. I believe in early delivery of orders so that the client has enough time to go through the work before submitting it. Am indeed the best option that any client that can think about.
4.50+
5+ Reviews
19+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Acetone (Table 8.2) has a significant dipole moment (2.7 D). Using structures, show the stabilizing interactions to be expected between acetone solvent molecules and (a) A dissolved potassium ion;...
-
A vessel filled with gas is divided into two equal parts I and 2 by a thin heat-insulating partition with two holes. One hole has a small diameter, and the other has a very large diameter (in...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
(1) How Does Strategy Respond to Environmental Factors Imprima Corporation is based in the United States and is examining the prospects for expanding into international markets with its main product,...
-
Is it really fair to assume that all business people are aware of these rules about exporting controlled items? What if an honest businessperson actually does not know about the laws?
-
What are the general criteria for eligibility for the earned income credit?
-
By using six factor formula for \(k\), derive the Eqs. (7.93), (7.94) of Section 7.7.1. dkoo dp= k MB dM dB 8 + (7.93) 1+M B M B2
-
Equity Securities Entries Capriati Corporation made the following cash purchases of securities during 2010, which is the first year in which Capriati invested in securities. 1. On January 15,...
-
1. Solve the following using convolution and correlation a. Let I={0, 0, 1, 0, 0} & Let k (mask) = {3, 2, 8} 3 3 b. Let I = & Let k (mask) = 3 3 1 24 3 4
-
Into a separatory funnel is poured 200 mL of dichloromethane (density = 1.33 g mL 1 ) and 55 mL of water. This mixture forms two layers. One milliliter of 2-octanol is added, and the mixture is...
-
In each case, which distribution has the higher entropy? Explain. (a) Four coins in which two are heads and two are tails, or four coins in which one is heads and three are tails. (b) Six coins in...
-
The management of Easterling Corp. is considering the effects of various inventory-costing methods on its financial statements and its income tax expense. Assuming that the price the company pays for...
-
Australia produces approximately 375 million kilograms of bananas annually and the vast majority of bananas are grown in northern Queensland. Australians consume 310 million kilograms of bananas at a...
-
Process manufacturing and services are classified into several categories.List and discuss the manufacturing processes and service classes for excellent operations management.
-
Real world work situations can result in some of the best learning experiences that we retain as life lessons. In an online environment, it can be difficult to pass on things with informal...
-
The pandemic has disrupted supply chains around the world, and in particular in the United States. Using your own independent research, explain what the supply chain is, its importance in the U.S....
-
How can my bank benefit from lowering the retail time accounts interest rates?
-
In 2005 General Motors (GM) announced that it would reduce employment by 30,000 workers. What does this decision reveal about how it viewed its marginal revenue product (MRP) and marginal resource...
-
Problem 2. (0.6 points, 0.2 points for each question) (a) A company turns its inventory 2 times a month. Its months-of-supply = Its days-of-supply = Please show your analysis below: _months. days. (1...
-
Predict the multiplicities of the absorptions for the hydrogen's of these groups, assume that hydrogen's labeled a are different from those labeled x but that all of those labeled a are identical and...
-
Predict the 1H.NMR spectra of these compounds include the approximate chemical shift, multiplicity, and integral for each type ofhydrogen. CI b) CH;CHCH; ) C,CH,H c) CH,CH,OCH,CH3 CH2CH2NO2 f)...
-
How many different absorption bands would appear in the 13C-NMR spectra of thesecompounds? b) CH;CH,CH,CH,CH3 c) CH CCH,CH;CH,CH, d)
-
What is the role and importance of labour law? Identify three events that were key to the evolution of labour law in Canada. ?
-
A lease owned by Company XYZ has to be evaluated. The lease is currently producing at a rate of 4000 Stb/month. It is estimated that production will be constant for one year, and then decline expe...
-
Analyze the labour law practice by the MNE(the home country)- attach the labour law ?

Study smarter with the SolutionInn App


