L-Rhamnose is a 6-deoxyaldose with the following structure. When a methyl glycoside of l-rhamnose, methyl a-l-rhamnopyranoside, was
Question:
L-Rhamnose is a 6-deoxyaldose with the following structure. When a methyl glycoside of l-rhamnose, methyl a-l-rhamnopyranoside, was treated with periodic acid, compound A, C6H12O5, was obtained that showed no evidence of a carbonyl group in its IR spectrum. Treatment of A with CH3I/Ag2O gave a derivative B, C8H16O5. Treatment of A with H2/Ni or NaBH4 gave compound C, shown here in Fischer projection. Give the structure of A. Explain why A gives no detectable carbonyl absorption in its IR spectrum, yet reacts with NaBH4.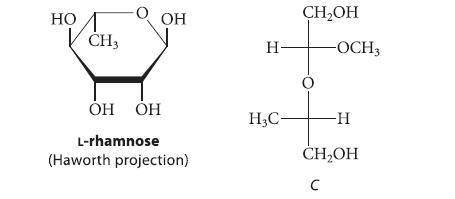
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





