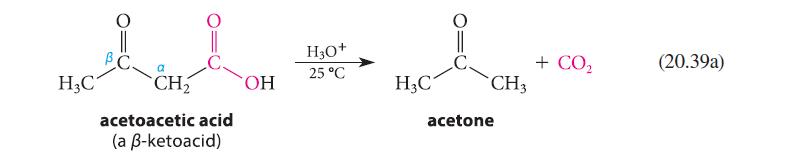
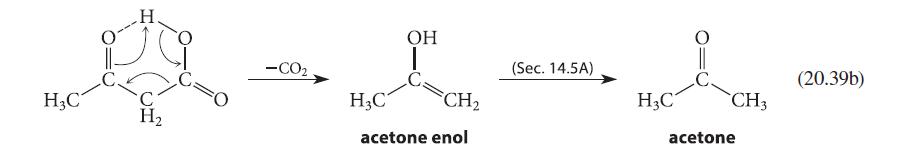
One piece of evidence supporting the enol mechanism in Eq. 20.39 is that b-keto acids that cannot
Question:
One piece of evidence supporting the enol mechanism in Eq. 20.39 is that βb-keto acids that cannot form enols are stable to decarboxylation. For example, the following b-keto acid can be distilled at 310°C without decomposition. Attempt to construct a model of the enol that would be formed when this compound decarboxylates. Use your model to explain why this b-keto acid resists decarboxylation.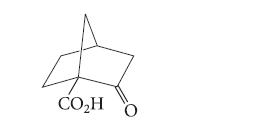
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





