The cis and trans stereoisomers of 4-chlorocyclohexanol give different products when they react with OH ,
Question:
The cis and trans stereoisomers of 4-chlorocyclohexanol give different products when they react with OH–, as shown in the reactions given in Fig. P9.83.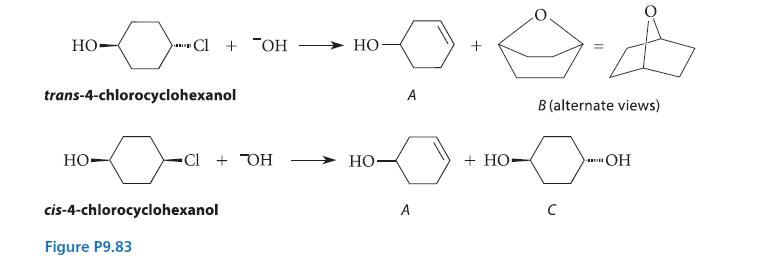
(a) Give a curved-arrow mechanism for the formation of each product.
(b) Explain why the bicyclic material B is observed in the reaction of the trans isomer, but not in the reaction of the cis isomer.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





