The following compound, unlike most phenols, is soluble in neutral aqueous solution, but insoluble in aqueous base.
Question:
The following compound, unlike most phenols, is soluble in neutral aqueous solution, but insoluble in aqueous base. Explain this unusual behavior.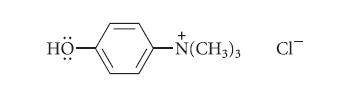
Transcribed Image Text:
HỌ -N(CH3)3 CI
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The solubility of most phenols in base due to their 1 charge when ionized Their c...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
When a pure substance is placed in contact with water, there are three possible outcomes. The substance may do nothing that is, the substance does not dissolve and no visible change takes place. The...
-
Toronto Tutors has been offering home tutoring services around the Toronto area since 2008. Roberta Draper started the business from her home, setting up tutoring sessions for math, science, and...
-
Part 1 a. Ammonia, NH 3 , is a weak electrolyte. It forms ions in solution by reacting with water molecules to form the ammonium ion and hydroxide ion. Write the balanced chemical reaction for this...
-
Consider again examples 9.2 and 9.3 , and once again assume that there are two states of the world for each random variable, denoted by V L j , V H j and n L , n H . Denote the probabilities for...
-
The time interval between the reception of the flashes at A and B in Problems 26 and 27 is 75 min according to the observer in S. How much time does he expect to have elapsed on the clock at A during...
-
Assume that the Financial Management Corporations $1,000-par-value bond had a 5.700% coupon, matured on May 15, 2020, had a current price quote of 97.708, and had a yield to maturity (YTM) of 6.034%....
-
What are the kernel-level units of execution in WinCE?
-
Moses Manufacturing is attempting to select the best of three mutually exclusive projects, X, Y, and Z. Although all the projects have 5-year lives, they possess differing degrees of risk. Project X...
-
Marie, a cash basis taxpayer, prepares an artist's tax return. She regularly charges $1,000 to prepare a tax return of that complexity. Because the artist was low on cash, Marie accepted one of his...
-
Given the structure of phenanthrene, draw structures of (a) 9,10-phenanthroquinone (b) 1,4-phenanthroquinone phenanthrene L 5 00 a N 10
-
Which of the two phenols in each set is more acidic? Explain. (a) 2,5-dinitrophenol or 2,4-dinitrophenol (b) phenol or m-chlorophenol (c) OH CH 0 or OH CH=0
-
The nuclear reaction that powers the sun is the fusion of four protons into a helium nucleus. The process involves several steps, but the net reaction is simply 4p 4 He + energy. The mass of a...
-
How is a swap similar to a forward contract?
-
Explain the rationale for reporting diluted earnings per share.
-
Suppose that Congress passes legislation that establishes a tax credit for small businesses and tax incentives for all businesses that invest in new plant and equipment. a) What is the anticipated...
-
Company K purchased $1 million face value of bonds issued by company J on 1 July 2015 at purchase cost of $1.05 million. The bond had coupon of 6% payable semi-annually and matures on 30 June 2017....
-
If Gemini has a floating rate loan and enters into a pay-fixed-and-receive-floating interest swap transaction with the same principal amount, which of the following statements is the most appropriate...
-
According to the U.S. Census Bureau, 20% of the workers in Atlanta use public transportation. If 25 Atlanta workers are randomly selected, what is the expected number to use public transportation?...
-
Research an article from an online source, such as The Economist, Wall Street Journal, Journal of Economic Perspectives, American Journal of Agricultural Economics, or another academic journal. The...
-
Each of the following descriptions applies to more than one alkane. In each case, draw and name two structures that match the description. (a) An isopropylheptane (b) A diethyldecane (c) A...
-
Give the IUPAC names of the following alkanes. (a) CH3C(CH3)2CH(CH)CH3)CH2CH2CH(CH3)2 (b) (c) (d) (e) (f) (g) (h) CH,CH CHCH CH, CH CH CH,CHCH CH,CHCH CH,CH CH,CH, CH, CH,CH, CH,CH,CH, C(CH,CH),...
-
Construct a graph, similar to Figure 3-11, of the torsional energy of 3-methylpentane along the C2-C3 bond. Place C2 in front, represented by three bonds coming together in a Y shape, and C3 in back,...
-
Assume the perpetual inventory system is used. Required: 1. Compute gross profit for the month of January for Laker Company for the four inventory methods. 2. Which method yields the highest gross...
-
"what is the role of the government interest in determining the constitutionality of government actions? Respond with referenced to atleast 3 substantive areas of constitutional law"
-
Data from the financial statements of Naranjo Co. and Jablonsky, Inc. are presented below (in millions): Naranjo Co. Jablonsky, Inc. Total liabilities, Year $73,044 $54,196 2 Total liabilities, Year...

Study smarter with the SolutionInn App


