The tetrabutylammonium salt of isotopically chiral phenyl phosphate was heated in the polar aprotic solvent acetonitrile containing
Question:
The tetrabutylammonium salt of isotopically chiral phenyl phosphate was heated in the polar aprotic solvent acetonitrile containing excess tert-butyl alcohol, and isotopically substituted tert-butyl phosphate was isolated. An analysis of its stereochemistry showed that it was completely racemic.
(See Fig. P25.31) Explain this result with a mechanism.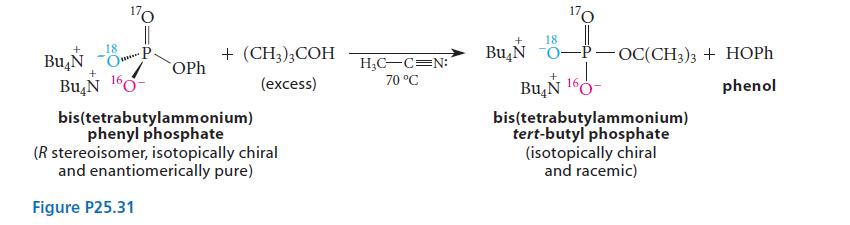
Transcribed Image Text:
+ Bu N 170 O P 18 **** + Bu N 160- OPh + (CH3)3COH (excess) bis(tetrabutylammonium) phenyl phosphate (R stereoisomer, isotopically chiral and enantiomerically pure) Figure P25.31 H₂C-C=N: 70 °C 18 Bu N 0- 170 -P- OC(CH3)3 + HOPh phenol Bu N 160- 160- bis(tetrabutylammonium) tert-butyl phosphate (isotopically chiral and racemic)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
Racemization of the product suggests that a single inve...View the full answer

Answered By

Rustia Melrod
I am a retired teacher with 6 years of experience teaching various science subjects to high school students and undergraduate students. This background enables me to be able to help tutor students who are struggling with the science of business component of their education. Teaching difficult subjects has definitely taught me patience. There is no greater joy for me than to patiently guide a student to the correct answer. When a student has that "aha!" moment, all my efforts are worth it.
The Common Core standards are a useful yardstick for measuring how well students are doing. My students consistently met or exceeded the Common Core standards for science. I believe in working with each student's individual learning styles to help them understand the material. If students were struggling with a concept, I would figure out a different way to teach or apply that concept. I was voted Teacher of the Year six times in my career. I also won an award for Innovative Teaching Style at the 2011 National Teaching Conference.
4.90+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Give the mechanism and major product for the reaction of a secondary haloalkane in a polar aprotic solvent with the following nucleophiles. The pKa value of the conjugate acid of the nucleophile is...
-
A sample containing an alkali sulfate is dried, weighed and dissolved in dilute HCl. Barium chloride solution is added in excess to precipitate barium sulfate, and the precipitate is digested in the...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
In 2020-21, a taxpayer makes a number of disposals, as listed below. Which of these disposals would be exempt from CGT? (a) An antique table sold for 5,000. (b) A watercolour painting sold at...
-
Why is a moderator needed in an ordinary nuclear fission reactor?
-
Kathy Snow wishes to purchase shares of Countdown Computing, Inc. The companys board of directors has declared a cash dividend of $0.80 to be paid to holders of record on Wednesday, May 12. a. What...
-
Let \(X_{t}=b t+W_{t}+Z_{t}\) where \(W\) is a Brownian motion and \(Z_{t}=\sum_{k=1}^{N_{t}} Y_{k}\) a \((\lambda, F)\)-compound Poisson process independent of \(W\). The first passage time above...
-
Melissa Mertz purchased a used tractor for $17,500. Before the tractor could be used, it required new tires, which cost $1,100, and an overhaul, which cost $1,400. Its first tank of fuel cost $75....
-
Company A is a well-known global brand that has been facing declining sales and loss of market share in recent years. As a brand manager, analyze the reasons behind this decline and propose...
-
The transition state for enzyme-catalyzed pyrophosphate hydrolysis, deduced from the structure of the enzyme inorganic pyrophosphatase, is shown in Fig. 25.6. (a) Indicate the catalytic role of each...
-
Describe the splitting expected in the proton resonance of the CH 2 group in triethyl phosphate. (The coupling constants for HH splitting and PH splitting happen to be identical in this case.)
-
Determine the interval(s) on which the following functions are continuous. Be sure to consider right- and left continuity at the endpoints. g(x) = x 4 - 1
-
For each scenario, determine which function of money is being described. a. Robert pays $8.00 to cross the Golden Gate Bridge. b. Liza considers which is the better deal: a desk lamp priced at...
-
What indicators should you use to track each of the following, and why? a. The overall size of the economy b. Labor market performance c. The future trajectory of economic activity d. Wages and...
-
Which of the following are signs of inflation? a. The price of a house in a high-demand market increased by 6% last year. b. CPI in the European Union was 106 in 2020 and 109 in 2021. c. The price of...
-
An economic downturn throws millions of people out of work. In some industries, workers who remain employed, or insiders, continue to develop their skills, enabling them to push wages above the level...
-
Javier is a department manager at a big box store. Over the last month sales have slumped and he has lots of inventory going unsold. Now its time to put in his orders to restock for next month. How...
-
Suppose a population proportion is .40, and 80% of the time when you draw a random sample from this population you get a sample proportion of .35 or more. How large a sample were you taking? Discuss.
-
Differentiate. y = ln(3x + 1) ln(5x + 1)
-
Draw the "enol" isomers of the following compound. (The "enol" of a nitro compound is called aci-nitro compound, and the "enol" of an amide is called an imidic acid.) Ph-C NH2 benzamide
-
Draw the s1r.uctufe of each of the following, compounds, (a) ten-butylamine (b) 2,2-dimethyl-3-hexanamine (c) N,N-diethy1-3-heptanamine
-
Design an enantiomeric resolution of racemic 2-phenylpropanoic acid using a pure enan-tiomer of 1-phenylethanamine as resolving agent. (assume that a solvent can be found in which the diasteromeric...
-
Ten years ago, the average amount of time a family spent eating supper together was 27.8 minutes. A sociologist believes this average has decreased. She randomly selects a sample of 12 families and...
-
A ball of mass m = 0.25 kg is moving in the x direction with an initial velocity of vo= 11 m/s. It strikes a ball of m = 2.1 kg initially at rest and sticks together. Find the final velocity for the...
-
Consider the non-dividend paying asset with a current value of 100 kr that is described by the 3-step Binomial model depicted below. Assume that the continuously compounded risk free interest rate is...

Study smarter with the SolutionInn App


