Compare the chemical shifts of the carbon atoms in the 13 C NMR spectra of the following
Question:
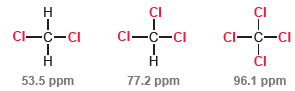
This is exactly what we would expect. Each chlorine atom withdraws electron density via induction, which deshields the carbon atom. The greater the number of chlorine atoms, the stronger the deshielding effect. However, when we compare the bromine analogues of the above compounds, we see the opposite trend:
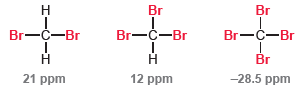
Can you offer an explanation for this anomalous trend? Consider the fact that a bromine atom is significantly larger than a chlorine atom and its electron density is spread over a larger volume of space.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





