Consider the following four energy diagrams: (a) Which diagrams correspond with a two-step mechanism? (b) Which diagrams
Question:
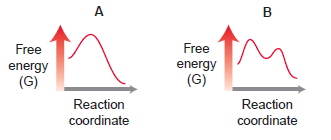
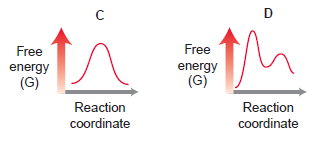
(a) Which diagrams correspond with a two-step mechanism?
(b) Which diagrams correspond with a one-step mechanism?
(c) Compare energy diagrams A and C. Which has a relatively larger Ea?
(d) Compare diagrams A and C. Which has a negative ΔG?
(e) Compare diagrams A and D. Which has a positive ΔG?
(f ) Compare all four energy diagrams. Which one exhibits the largest Ea?
(g) Which processes will have a value of Keq that is greater than 1?
(h) Which process will have a value of Keq that is roughly equal to 1?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





