In the previous problem, we saw that an acetylide ion can attack a variety of electrophiles. In
Question:
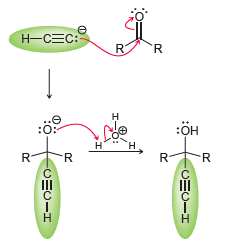
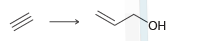
The acetylide ion attacks the ketone, generating an alkoxide ion. After the reaction is complete, a proton source is used to protonate the alkoxide ion. In a synthesis, these two steps must be shown separately, because the acetylide ion will not survive in the presence of H3O+. Using this information, propose a plausible synthesis for allyl alcohol, using acetylene as your only source of carbon atoms:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





