Single bonds generally experience free rotation at room temperature: Nevertheless, the single bond shown below exhibits a
Question:
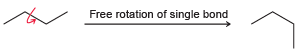
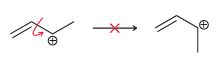
Nevertheless, the €œsingle bond€ shown below exhibits a large barrier to rotation. In other words, the energy of the system is greatly increased if that bond is rotated. Explain the source of this energy barrier. (Hint: Think about the atomic orbitals being used to form the €œconduit.€)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





