The following reaction exhibits a second-order rate equation: a) What happens to the rate if the concentration
Question:
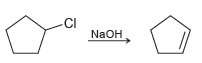
a) What happens to the rate if the concentration of chlorocyclopentane is tripled and the concentration of sodium hydroxide remains the same?
b) What happens to the rate if the concentration of chlorocyclopentane remains the same and the concentration of sodium hydroxide is doubled?
c) What happens to the rate if the concentration of chlorocyclopentane is doubled and the concentration of sodium hydroxide is tripled?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





