Question: We saw in Section 25.6 that DCC can be used to form a peptide bond. We explored the mechanism, and we saw that DCC activates
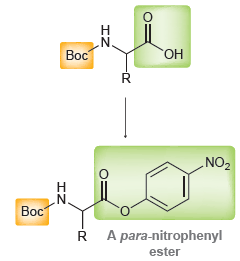
The activated ester is readily attacked by a suitably protected amino acid to form a peptide bond:
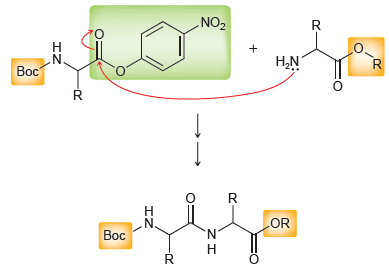
(a) Explain how the p-nitrophenyl ester activates the carbonyl group toward nucleophilic acyl substitution.
(b) What is the function of the nitro group?
(c) A meta-nitrophenyl ester is less activating than a paranitrophenyl ester. Explain.
" Boc NO2 Boc A para-nitrophenyl ester R. NO2 .N Boc LOR N. Boc R. R-
Step by Step Solution
3.29 Rating (173 Votes )
There are 3 Steps involved in it
a The COOH group does not readily undergo nucleophilic acyl sub... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
1625_606b0df1d8630_662501.pdf
180 KBs PDF File
1625_606b0df1d8630_662501.docx
120 KBs Word File


