Question: A box like the one in Problem 31 has two particles in compartment A, two particles in compartment B, and four energy units to be
A box like the one in Problem 31 has two particles in compartment A, two particles in compartment B, and four energy units to be distributed.
(a) Construct a table like Table 19. 2 (page 656) for this setup.
(b) Does equipartition of energy take place even with such a small number of particles?
Data from Problem 31
A box divided into compartments \(A\) and \(B\) contains 14 particles in \(A\) and 6 in \(B\). The separating partition allows energy exchange between compartments as the particles collide with the partition. Table 19. 2 (page 656) lists the numbers of basic states possible when 10 energy units are distributed in various combinations over the 20 particles. How much more likely is it for compartment \(A\) to contain 7 energy units (macrostate \(E_{\mathrm{A}}=7, E_{\mathrm{B}}=3\) ) than for this compartment to contain 3 energy units (macrostate \(E_{\mathrm{A}}=3, E_{\mathrm{B}}=7\) )?
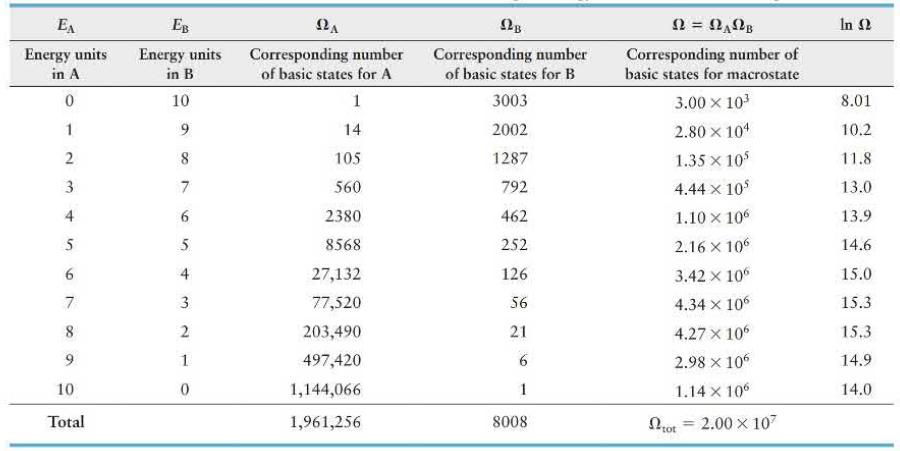
EA Energy units EB Energy units in B Corresponding number of basic states for A Corresponding number of basic states for B Corresponding number of basic states for macrostate In in A 0 10 1 3003 3.00 103 8.01 1 9 14 2002 2.80 104 10.2 2 8 105 1287 1.35 105 11.8 3 7 560 792 4.44 105 13.0 4 6 2380 462 1.10 x 106 13.9 5 5 8568 252 2.16 x 106 14.6 6 4 27,132 126 3.42 x 106 15.0 7 3 77,520 56 4.34 x 106 15.3 8 9 10 210 203,490 21 4.27 x 106 15.3 497,420 6 2.98 106 14.9 0 1,144,066 1 1.14 x 106 14.0 Total 1,961,256 8008 Lot = 2.00 107
Step by Step Solution
3.33 Rating (156 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


