Question: (a) Does the negative change in entropy in Example 19.12 violate the Eq. 19.5 part of the entropy law? (b) What is the change in
(a) Does the negative change in entropy in Example 19.12 violate the Eq. 19.5 part of the entropy law?
(b) What is the change in the thermal energy of the gas in Example 19.12?
Data from Example 19.12
A sample of a monatomic ideal gas consisting of \(1.00 \times 10^{23}\) atoms in a volume of \(4.00 \times 10^{-3} \mathrm{~m}^{3}\) is initially at a temperature of \(290 \mathrm{~K}\). The gas is cooled until it reaches a pressure of \(5.00 \times 10^{4} \mathrm{~N} / \mathrm{m}^{2}\).
What is the change in entropy for the gas and for each atom?
What is the ratio of the final rms speed of each atom to its initial rms speed?
How does the change in the entropy of the gas depend on this ratio \(v_{\text {rms, }, \mathrm{f}} / v_{\text {rms, } \mathrm{i}}\) ?
Eq. 19.5.
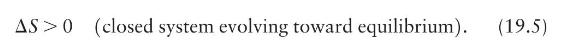
AS 0 (closed system evolving toward equilibrium). (19.5)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


