Question: In an atom, what limits the separation between the electron cloud and the nucleus in the presence of an external charge? Why, for example, isn't
In an atom, what limits the separation between the electron cloud and the nucleus in the presence of an external charge? Why, for example, isn't the electron cloud in Figure \(22.20 b\) pulled all the way to the location of the external positive charge?
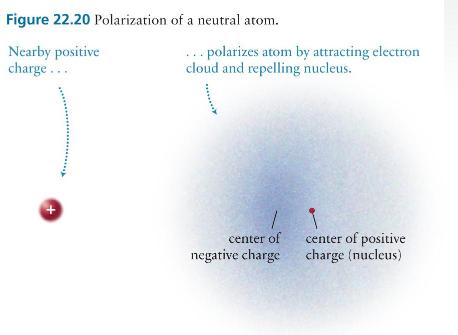
Figure 22.20 Polarization of a neutral atom. Nearby positive charge... ... polarizes atom by attracting electron cloud and repelling nucleus. + center of negative charge center of positive charge (nucleus)
Step by Step Solution
3.37 Rating (144 Votes )
There are 3 Steps involved in it
The electron cloud is attracted not only to the ... View full answer

Get step-by-step solutions from verified subject matter experts


