Question: Which material in Table 20. 2 has the greatest specific heat capacity? The smallest? Imagine that objects made of these materials are placed on your
Which material in Table 20. 2 has the greatest specific heat capacity? The smallest? Imagine that objects made of these materials are placed on your hand. The objects have the same mass and are initially at the same temperature. Each object equilibrates thermally with your hand, which is at a higher temperature. Describe any differences in the equilibration process.
Data from Table 20. 2
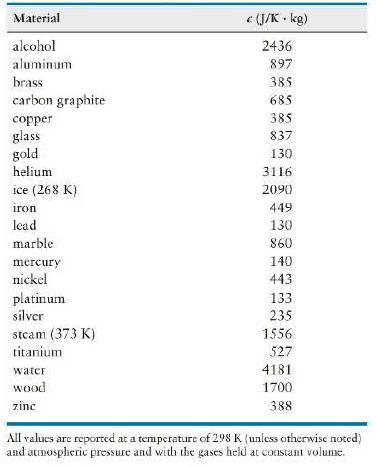
Material (J/K. kg) alcohol 2436 aluminum 897 brass 385 carbon graphite 685 copper 385 glass 837 gold 130 helium 3116 ice (268 K) 2090 iron 449 lcad 130 marble 860 mercury 140 nickel 443 platinum 133 silver 235 steam (373 K) 1556 titanium 527 water 4181 wood 1700 zinc 388 All values are reported at a temperature of 298 K (unless otherwise noted) and atmospheric pressure and with the gases held at constant volume.
Step by Step Solution
3.38 Rating (151 Votes )
There are 3 Steps involved in it
Water is the highest gold and lead the lowest Because water has such a high ... View full answer

Get step-by-step solutions from verified subject matter experts


