Benzene, C 6 H 6 , belongs to the D 6h group. The reducible representation for the
Question:
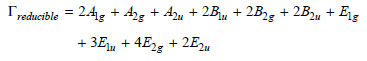
a. How many vibrational modes does benzene have?
b. How many of these modes are infrared active and to which representation do they belong?
c. Which of the infrared active modes are degenerate in energy and what is the degeneracy for each?
d. How many of these modes are Raman active and to which representation do they belong?
e. Which of the Raman active modes are degenerate in energy and what is the degeneracy for each?
f. Which of the infrared modes are also Raman active?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





