Fifty milliliters of 100% H 2 SO 4 at 25C and 84.2mL of liquid water at 15C
Question:
Fifty milliliters of 100% H2SO4 at 25°C and 84.2mL of liquid water at 15°C are mixed. The heat capacity of the product solution is 2.43 J/(g °C).
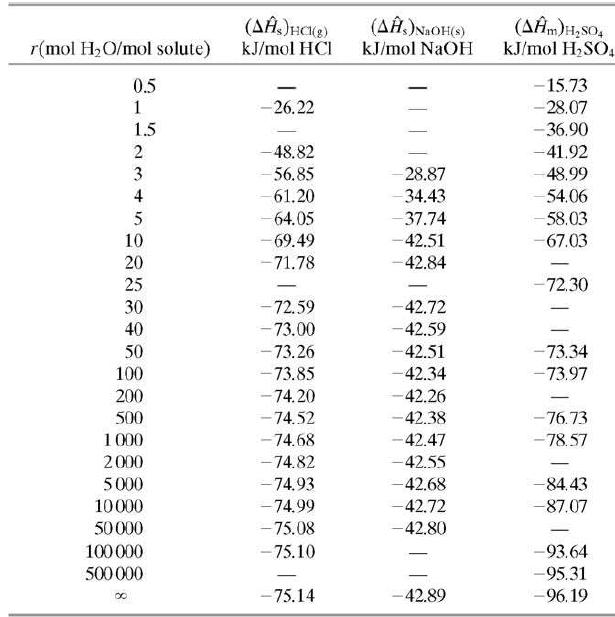
(a) Estimate the maximum temperature attainable by the product solution and state the conditions under which this temperature would be attained, using heat of mixing data from Table B.11.
(b) Give several reasons why the temperature calculated in Part (a) could not be attained in practice.
(c) Estimate how much heat would have to be transferred from the mixing vessel to keep the temperature of the product solution at 25°C. (You should be able to solve this problem quickly by looking back at the enthalpy table of Part (a).)
Table B.11
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





