Two hundred kilograms per hour of an aqueous solution containing 20.0 mole% sodium acetate (NaC2H3O2) enters an
Question:
Two hundred kilograms per hour of an aqueous solution containing 20.0 mole% sodium acetate (NaC2H3O2) enters an evaporative crystallizer at 60°C. When the solution is exposed to the low pressure in the evaporator, 16.9% of the water evaporates, concentrating the remaining solution and causing crystals of sodium acetate trihydrate (NaC2H3O2 ∙ 3H2O) to form. The product is an equilibrium mixture of crystals and a saturated aqueous solution containing 15.4 mole% NaC2H3O2.
The effluents (crystals, solution, and water vapor) are all at 50°C.
(a) Calculate the feed rate to the crystallizer in kmol/h.
(b) Calculate the production rate (kg/h) of trihydrate crystals and the mass flow rate (kg/h) of the liquid solution in which the crystals are suspended.
(c) Estimate the rate (kJ/h) at which heat must be transferred to or from the crystallizer (state which), using the following physical property data:

Heat of solution of anhydrous sodium acetate:
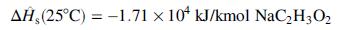
Heat of hydration:
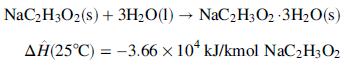
(d) In a commercial process to produce crystalline sodium acetate trihydrate, what are several processing steps the slurry leaving the crystallizer might undergo?
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





