From the following data at 298.15 K as well as data in Table 4.1 (Appendix B, Data
Question:
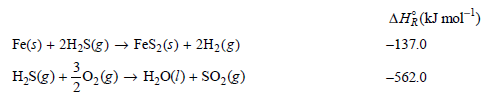
Transcribed Image Text:
AĦR(kJ mol) Fe(s) + 2H2S(g) –→ FeS2(s) + 2H2(g) -137.0 H;S(g) + 0,(g) → H,O() + SO,(E) -562.0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
HOl SOg HSg 20 8 Ss Og SO g Hg Og HO1 ...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the standard enthalpy of formation for diamond, given that C(graphite) + O2(g) CO2(g) Afr =-393.5 kJ/mol C(diamond) + O2(g) CO2(g) AF1 395.4 kJ/mol
-
From the following data at 298.15 K calculate the standard enthalpy of formation of FeO(s) and of Fe 2 O 3 (s): A(kJ mol) Fe,0;(s) + 3C(graphite) 2Fe(s) + 3cO(g) FeO(s) + C(graphite) Fe(s) + CO(g)...
-
From the enthalpy of formation for CO2 and the following information, calculate the standard enthalpy of formation for carbon monoxide (CO). Why can't we obtain it directly by measuring the enthalpy...
-
The following items are dropped from an airplane. Rank them in order from lowest terminal speed to highest and justify your ranking. (a) Bowling ball (b) Beach ball (c) Spear or javelin (pointing...
-
San Antonio Company produces accounting software. Its unit cost structure, based on an anticipated production volume of 150,000 units, is:sale price is 160, variable costs is 60 and fixed costs is...
-
Differentiate among conflict, collaboration, and competition.
-
Consider the following "case-control" sample selection method for binary dependent variables. Intuitively, if we are working with a problem in which the event of interest is rare, we want to make...
-
DeSoto Tools Inc. is planning to expand production. The expansion will cost $300,000, which can be financed either by bonds at an interest rate of 14 percent or by selling 10,000 shares of common...
-
In the past, 30% of a country club's members brought guests to play golf sometime during the year. Last year, the club initiated a new program designed to encourage members to bring more guests to...
-
Candidates for political office realize that different levels of support among men and women may be a crucial factor in determining the outcome of an election. One candidate finds that 52% of 473 men...
-
Which of Ne or Ar has the larger van der Waals parameter a? Explain your reasoning.
-
Which of Ne or Ar has the larger van der Waals parameter b? Explain your reasoning.
-
Green Wave Company plans to own and operate a storage rental facility. For the first month of operations, the company has the following transactions. 1. Issue 10,000 shares of common stock in...
-
you want to have 10,000 seven years from now how much will you have to save each month if the annual interest is 6% compounded monthly
-
A company's actual assessable payroll is $735,250.00. The premium rate is $1.35 per $100 of assessable payroll. Calculate the company's annual worker's compensation assessment.
-
74f12) 78: regulon function of Z. P.T DR +34=4
-
How important is a college education for social mobility in the United States? Why? Imagine you were accepted into the best school in the country, but attending meant you would graduate with $120,000...
-
When is 100% of a warrant or right taxed as a capital gain?
-
What do we call two colors that add to produce white?
-
As indicated by mutual fund flows, investors tend to beat the market seek safety invest in last year's winner invest in last years loser
-
A lot of information about the energy levels and wave functions of small inorganic molecules can be obtained from their ultraviolet spectra. An example of a spectrum with considerable vibrational...
-
Aromatic hydrocarbons and 12 form complexes from which charge transfer electronic transitions are observed the hydrocarbon acts as an electron donor and 12 as an electron acceptor the energies hvmax...
-
Consider some of the precautions that must be taken when conducting single-molecule spectroscopy experiments. (a) What is the molar concentration of a solution in which there is, on average, one...
-
Research the different processes involved during the planning phase of a project. Identify the tasks and techniques associated with each of these processes. Prepare diagram where you relate...
-
How do cultural norms and values intersect with social structure, influencing patterns of behavior, identity formation, and societal organization ?
-
We manufacture breakfast cereal at our factory in Toad Suck, AR. Our current selling price is $2.40 per box, F.O.B. our shipping dock (our customers pay shipping expenses). Our gross profit margin is...

Study smarter with the SolutionInn App


