The vapor pressure of ethanol(l) is given by a. Calculate the standard boiling temperature. b. Calculate ÎH
Question:
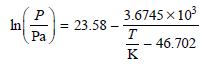
a. Calculate the standard boiling temperature.
b. Calculate ΔH vaporization at 298 K and at the standard boiling temperature.
Transcribed Image Text:
3.6745 x 10 23.58 In Pa – - 46.702 K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 61% (13 reviews)
a In b Pa 20767 ...View the full answer

Answered By

Hamza Amjad
Currently I am student in master degree program.from last two year I am tutring in Academy and I tought many O/A level student in home tution.
4.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The vapor pressure of an unknown solid is approximately given by ln(P/Torr) = 22.413 2211 (K/T), and the vapor pressure of the liquid phase of the same substance is approximately given by ln(P/Torr)...
-
Carbon tetrachloride melts at 250. K. The vapor pressure of the liquid is 10,539 Pa at 290. K and 74,518 Pa at 340. K. The vapor pressure of the solid is 270. Pa at 232 K and 1092 Pa at 250. K. a....
-
Consider the process which is carried out at constant pressure. The total DS for this process is known to be 75.0 J K-1mol-1. For A(l) and A(g), the Cp values are 75.0 JK-1mol-1 and 29.0 JK-1mol-1,...
-
8 for 0 < < 6 for 6
-
Discus the Ten Strategies of a World-Class Computer Security Incident Response Team, Research and find at least three (3) more recommendations needed to organize, fund and introduce a CSIRT. Research...
-
If two genes are found on the same chromosome, are they always inherited together? Why or why not?
-
A gray gas with an absorption coefficient of \(\kappa\) is contained between two plates separated by a distance \(L\). Using the diffusion approximation for radiation, derive the relation...
-
Use the Rolling Hills, Inc. data from Problem P14-34A. Requirements 1. Prepare the 2018 statement of cash flows by the direct method. 2. How will what you learned in this problem help you evaluate an...
-
Merchandise inventory Store supplies 33,600 1,915 975 Office supplies Prepaid insurance 5,255 Equipment 63,490 Accumulated depreciation, equipment $ 12,655 Accounts payable 7,000 Salaries payable 0...
-
Susie Smith opened Susie's Commerical Clearning on April 1, 2021. In Apr, the following transactions were completed. Apr-01 Invested $14,000 cash in the business 1 Purchased a used truck for $26,400,...
-
Use the vapor pressures of tetrachloromethane given in the following table to calculate the enthalpy of vaporization using a graphical method or a least squares fitting routine. P/Pa T (K) 320. 330....
-
In Section 8.8, it is stated that the maximum height of a water column in which cavitations does not occur is 9.7 m. Show that this is the case at 298 K.
-
The unadjusted trial balance of Good Times, Inc., at January 31, 2010, does not balance. In addition, the trial balance needs to be adjusted before the financial statements at January 31, 2010 can be...
-
Select a specific service product you are familiar with and identify its core product and supplementary services. Select a competing service and analyze the differences between the two in terms of...
-
Using a firm you are familiar with, analyze what opportunities it might have to create product line extensions for its current and/or new markets. What impact might these extensions have on its...
-
Do the 3 Cs and STP apply to digital services and platforms as well? Is the way positioning works for these services different from that of other services?
-
The first version of a website for a financial services company has been live for a year. Originally it was developed by a team of two people, and was effectively brochureware. The second version of...
-
How should social media marketing effectiveness be assessed?
-
A string of length L is initially stretched into a zigzag profile, with linear segments of string connecting the (x, f(x)) points (0, 0), (L/4, a), (3L/4,a), (L, 0). Compute the Fourier series...
-
Copy and complete the statement. 3800 m ? km =
-
Find the 3 by 3 matrix that is equivalent in its action to each of the symmetry operators: (a) S 2(z) . (b) C 2(x) .
-
Find S 2(y) (3,4,5).
-
Find the result of each operation on the given point (represented by Cartesian coordinates): (a) C 2(z) i (1,1,1). (b) i C 2(z) (1,1,1).
-
Problem: Module 3 Textbook Problem 5 Learning Objective: 3-6 Using the straight-line method show how bonds issued at a discount. affect financial statements Diaz Company issued $91,000 face value of...
-
Manvir had to make payments of $1,125 every 6 months to settle a $22,000 loan that he received at 4.52% compounded semi-annually. a. How long did it take to settle the loan?
-
If I invest a single amount of $14,000 in an account earning 8% p.a. compounding quarterly for 5 years, how much interest will I have earned in those 5 years?

Study smarter with the SolutionInn App


