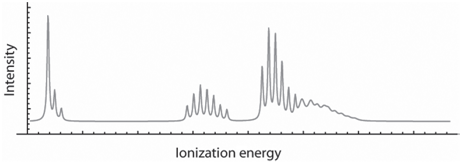
Suppose you obtain the UV photoelectron spectrum shown here for a gas-phase molecule. Each of the groups
Question:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





