Question: The angular functions, Î(θ )Φ(Ï ), for the one-electron HartreeFock orbitals are the same as for the hydrogen atom, and the radial functions and radial
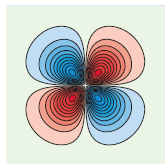
The angular functions, Θ(θ )Φ(φ ), for the one-electron Hartree€“Fock orbitals are the same as for the hydrogen atom, and the radial functions and radial probability functions are similar to those for the hydrogen atom. The contour coloring is explained in the caption to Figure 20.7. The following figure shows:
a. A contour plot in the xy plane with the y axis being the vertical axis,
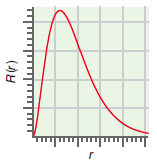
b. The radial function,
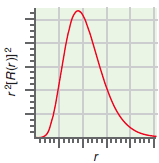
c. The radial probability distribution for a one-electron orbital. Identify the orbital (2s, 4dxz, and so on).
T (
Step by Step Solution
3.46 Rating (172 Votes )
There are 3 Steps involved in it
a b c This orbita... View full answer

Get step-by-step solutions from verified subject matter experts


