Question: The following temperaturecomposition data were obtained for a mixture of octane (O) and methylbenzene (M) at 1.00 atm, where x is the mole fraction in
The following temperature–composition data were obtained for a mixture of octane (O) and methylbenzene (M) at 1.00 atm, where x is the mole fraction in the liquid and y the mole fraction in the vapour at equilibrium.
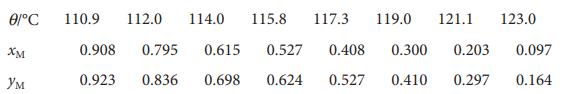
The boiling points are 110.6 °C and 125.6 °C for M and O, respectively. Plot the temperature–composition diagram for the mixture. What is the composition of the vapour in equilibrium with the liquid of composition (i) xM =0.250 and (ii) xO =0.250?
0/ 110.9 112.0 114.0 115.8 117.3 119.0 121.1 123.0 0.908 0.795 0.615 0.527 0.408 0.300 0.203 0.097 0.923 0.836 0.698 0.624 0.527 0.410 0.297 0.164 XM
Step by Step Solution
3.43 Rating (169 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


