Many chemical reactions are the result of the interaction of two molecules that undergo a change to
Question:
Many chemical reactions are the result of the interaction of two molecules that undergo a change to produce a new product. The rate of the reaction typically depends on the concentrations of the two kinds of molecules. If a is the amount of substance A and b is the amount of substance B at time t = 0, and if x is the amount of product at time t, then the rate of formation of x may be given by the differential equation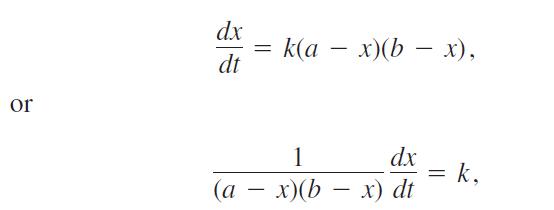
where k is a constant for the reaction. Integrate both sides of this equation to obtain a relation between x and t
(a) If a = b, and
(b) If a ≠ b. Assume in each case that x = 0 when t = 0.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Thomas Calculus Early Transcendentals
ISBN: 9780321884077
13th Edition
Authors: Joel R Hass, Christopher E Heil, Maurice D Weir
Question Posted:





