Question: Color Plate 4 shows how the color of the acid-base indicator bromocresol green (H 2 BG) changes as NaCl is added to an aqueous solution
Color Plate 4 shows how the color of the acid-base indicator bromocresol green (H2BG) changes as NaCl is added to an aqueous solution of (H+)(HBG-). Explain why the color changes from pale green to pale blue as NaCl is added.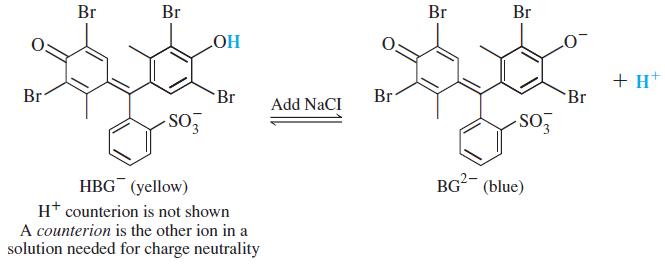
Br Br Br Br + H* Br Br Br Br Add NaCI -SO BG (blue) HBG (yellow) H* counterion is not shown A counterion is the other ion in a solution needed for charge neutrality
Step by Step Solution
3.54 Rating (161 Votes )
There are 3 Steps involved in it
On addition of NaCl the ionisabl... View full answer

Get step-by-step solutions from verified subject matter experts


