Buoyancy correction factor: mtrue/mread = 1.000 3244] [Interpolated density of water at 23.3 C: 0.997 468 9
Question:
Buoyancy correction factor: mtrue/mread = 1.000 3244]
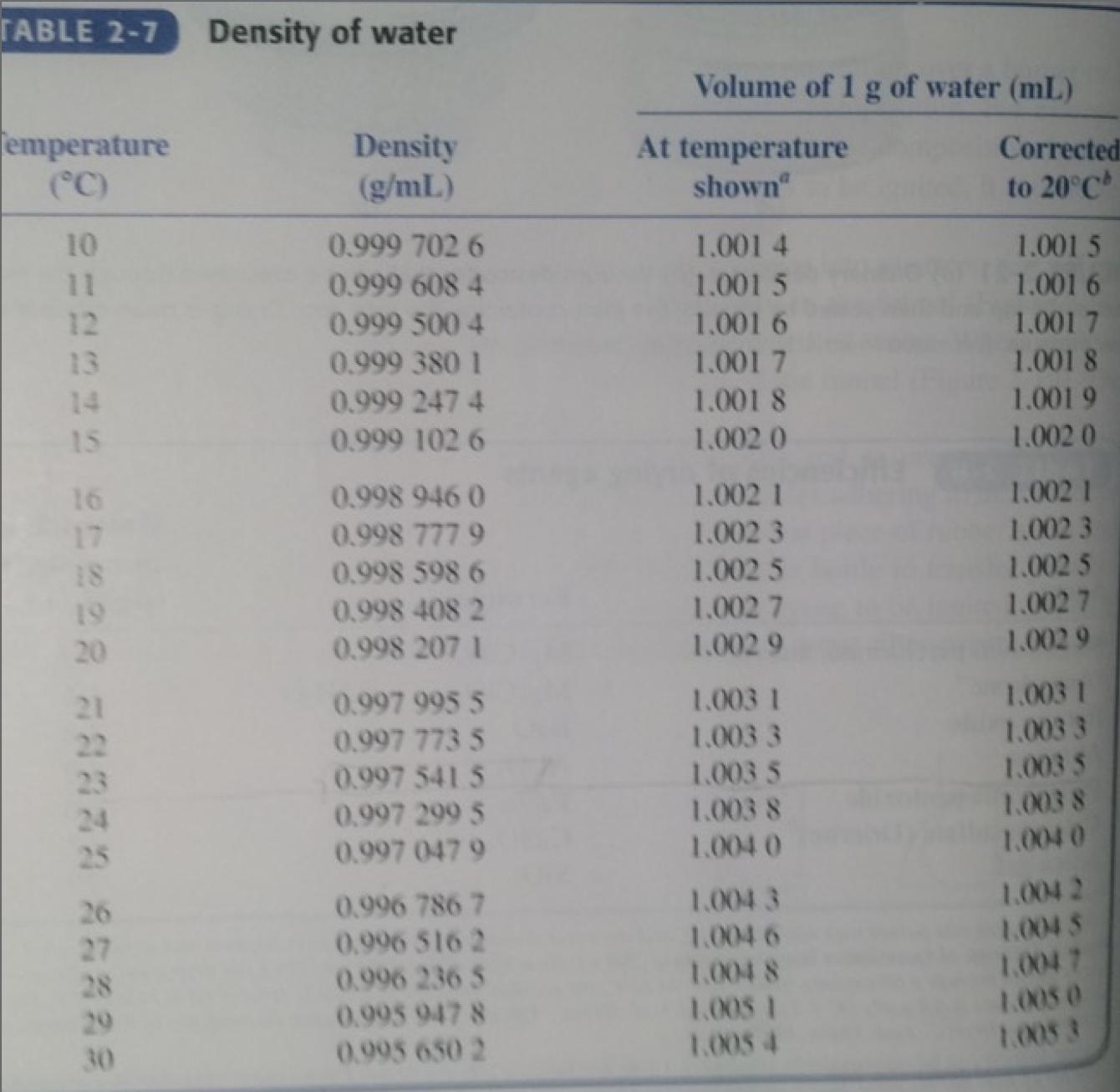
[Interpolated density of water at 23.3 °C: 0.997 468 9 g/mL]
[HCl concentration at 20 °C: 0.102343 M]
Reagents:
The molar mass and density of Na2CO3 are given
-dry, primary standard Na2CO3 (jars in 110 °C oven or cooling in desiccators) [Na2CO3: (105.98833 ± 0.00156) g/mol; d = 2.53 g/mL]
-bromocresol green indicator solution [0.1 g(sodium salt)/ 250 mL H20]
~0.1 M HCl to be standardized
Please show your full calculation of the buoyancy correction factor with the sample problem. Always calculate buoyancy correction factors to at least 5 decimal places. Please show your full calculation for linear interpolation of water densities as well. Make sure the answers exactly match the ones given below. We will often include extra guard digits to help you check this.
SAMPLE PROBLEM: A 0.1355 g sample (uncorrected mass) of dry, primary-standard Na2CO3 required 25.05 mL of HCl solution (at 23.3 °C) to reach the end point. A blank titration with the same amount of indicator required 0.04 mL. Use linear interpolation and the data in Table 2-7 of Harris to estimate the density of water at 23.3 °C. What is the concentration of the HCl solution, corrected to 20 °C?
Step by Step Answer:






