The oxidation numbers of Cu and Bi in high-temperature superconductors of the type Bi 2 Sr 2
Question:
The oxidation numbers of Cu and Bi in high-temperature superconductors of the type Bi2Sr2(Ca0.8Y0.2)Cu2Ox (which could contain Cu2+, Cu3+, Bi3+, and Bi5+) can be measured by the following procedure. In Experiment A, the superconductor is dissolved in 1 M HCl containing excess 2 mM CuCl. Bi5+(written as BiO3- ) and Cu3+ consume Cu+ to make Cu2+:
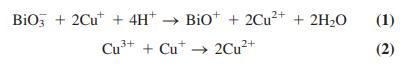
The excess, unreacted Cu+ is then titrated by coulometry (described in Chapter 16). In Experiment B, the superconductor is dissolved in 1 M HCl containing excess 1 mM FeCl2•4H2O. Bi5+ reacts with the Fe2+ but Cu3+ does not react with Fe2+
.png)
The excess, unreacted Fe2+ is then titrated by coulometry. The total oxidation number of Cu+ Bi is measured in Experiment A, and the oxidation number of Bi is determined in Experiment B. The difference gives the oxidation number of Cu.
(a) In Experiment A, a sample of Bi2Sr2CaCu2Ox (FM 760.37 15.999 4x) (containing no yttrium) weighing 102.3 mg was dissolved in 100.0 mL of 1 M HCl containing 2.000 mM CuCl. After reaction with the superconductor, coulometry detected 0.108 5 mmol of unreacted Cu in the solution. In Experiment B, 94.6 mg of superconductor were dissolved in 100.0 mL of 1 M HCl containing 1.000 mM FeCl2•4H2O. After reaction with the superconductor, coulometry detected 0.057 7 mmol of unreacted Fe2. Find the average oxidation numbers of Bi and Cu in the superconductor and the oxygen stoichiometry coefficient, x.
(b) Find the uncertainties in the oxidation numbers and x if the quantities in Experiment A are 102.3 (±0.2) mg and 0.108 5 (±0.000 7) mmol and the quantities in Experiment B are 94.6 (±0.2) mg and 0.057 7 (±0.000 7) mmol. Assume negligible uncertainty in other quantities.
Step by Step Answer:






