Question: (a) From Kw in Table 6-1, calculate the pH of pure water at 0, 20, and 40C. (b) For the reaction D 2 O
(a) From Kw in Table 6-1, calculate the pH of pure water at 0°, 20°, and 40°C.
(b) For the reaction D2O ⇌ D+ + OD-, K = [D+][OD-] =1.35 × 10-15 at 25°C. In this equation, D stands for deuterium, which is the isotope 2H. What is the pD (= –log[D+]) for neutral D2O?
Table 6-1
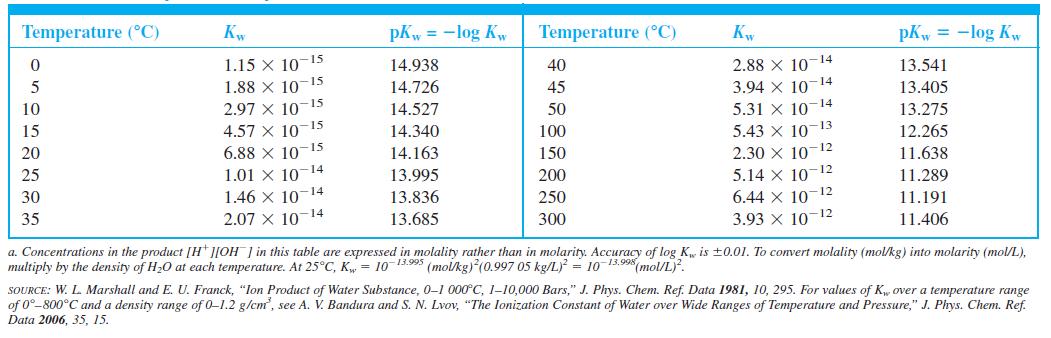
Temperature (C) Kw pKw = -log Kw Temperature (C) pKw = -log Kw 15 2.88 x 10-14 3.94 X 10 5.31 X 10 5.43 x 10-13 2.30 X 10 12 5.14 X 10-12 6.44 X 10 3.93 X 10-12 1.15 X 10 14.938 40 13.541 1.88 X 10-15 2.97 X 10 4.57 X 10 6.88 X 10-15 14 14.726 45 13.405 15 14 10 14.527 50 13.275 15 15 14.340 100 12.265 20 14.163 150 11.638 1.01 x 10-14 1.46 x 10-14 2.07 X 1014 25 13.995 200 11.289 12 30 13.836 250 11.191 35 13.685 300 11.406 a. Concentrations in the product [H* ][OH ] in this table are expressed in molality rather than in molarity. Accuracy of log Kw is 0.01. To convert molality (mol/kg) into molarity (mol/L), multiply by the density of H20 at each temperature. At 25C, K = 10-13.995 (molkg) (0.997 05 kg/L) = 10 13.998(mol/L. SOURCE: W. L. Marshall and E. U. Franck, "lon Product of Water Substance, 0-1 000C, 110,000 Bars," J. Phys. Chem. Ref. Data 1981, 10, 295. For values of K over a temperature range of 0-800C and a density range of 0-1.2 g/cm, see A. V. Bandura and S. N. Lvov, "The lonization Constant of Water over Wide Ranges of Temperature and Pressure," J. Phys. Chem. Ref. Data 2006, 35, 15.
Step by Step Solution
3.40 Rating (162 Votes )
There are 3 Steps involved in it
Kw Ionic product of water ... View full answer

Get step-by-step solutions from verified subject matter experts


