Histidine is a triprotic amino acid: What is the value of the equilibrium constant for the reaction
Question:
Histidine is a triprotic amino acid:
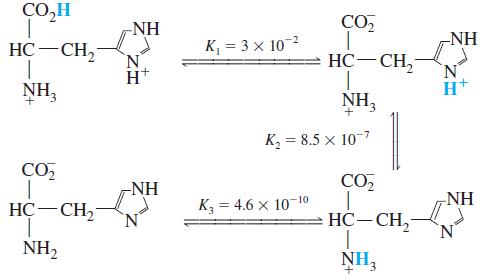
What is the value of the equilibrium constant for the reaction
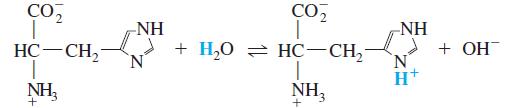
Transcribed Image Text:
CO,H -NH CO, -NH HC-CH, K, = 3 x 102 HC-CH, NH, NH, к, 3 8.5 х 10-7 CO, -NH CO NH HC-CH2 К, 3 4.6 х 10 10 HC-CH, N. NH2 NH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 37% (8 reviews)
Using definition of equilibrium constant Loo...View the full answer

Answered By

Amit Kulhria
I have been working as Chemistry professor while training students for JEE Main and Advanced for about 7 years. Besides I have taught general science to Civil services aspirants.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Write the stepwise acid-base reactions for the following ions in water. Write the correct symbol (for example, Kb1) for the equilibrium constant for each reaction. a. b. H3NCH2CH2NH...
-
The amino acid glycine (H2N-CH2-COOH) can participate in the following equilibria in water: (a) Use the values of Ka and Kb to estimate the equilibrium constant for the intramolecular proton transfer...
-
What average yield per amino acid would be required to synthesize a protein containing 100 amino acids in 507% overall yield?
-
Explain the concept of recursion in programming and provide an example of a recursive function.
-
How are Treasury regulations and revenue rulings different?
-
Write, test, validate, and debug (if necessary) markup documents and JavaScript files for the following: Modify the document of Exercise 6.9 to make the statement change color between red and blue...
-
How and why does the architecture of the information system differ from software architecture?
-
Below are listed various costs that are found in organizations. 1. Hamburger buns in a Wendys outlet. 2. Advertising by a dental office. 3. Apples processed and canned by Del Monte. 4. Shipping...
-
(b) What risk factors might pertain to a private equity investment in the pandemic? (9 marks) (c) Explain how a MBO deal is usually structured both in terms of its corporate/legal structure and its...
-
A regulation basketball is initially flat and is then inflated to a pressure of approximately 24 lb/in absolute. Consider the air temperature to be constant at 70 F. Find the mass of air required to...
-
Succinic acid dissociates in two steps: K1 H,H,C ,, + H* %3| || OCCH,CH,CO + H* , 3 2.3 10-6 HOCCH,CH,CO Calculate Kp1 and Kp2 for the following reactions: || OCCH,CH,CO + H,0 = HOCCH,CH,CO + OH ...
-
(a) From Kw in Table 6-1, calculate the pH of pure water at 0, 20, and 40C. (b) For the reaction D 2 O D + + OD - , K = [D + ][OD - ] =1.35 10 -15 at 25C. In this equation, D stands for deuterium,...
-
Aurora National Bank has two service departments, the Human Resources (HR) Department and the Computing Department. The bank has two other departments that directly service customers, the Deposit...
-
If traditional FFS leads to demand inducement, what constrains the HMO from underproviding care?
-
Suppose that the licensure requirements for health care providers were eliminated. Use supply-and-demand analysis to predict what may happen to the price and quantity of health care services. Are...
-
If there were no subsidies for medical education, would enrollments be larger or smaller? Would the return to medical education be larger or smaller? If physician education was not subsidized, would...
-
Some argue that wide disparities in utilization rates across racial and ethnic groups are indicative of discrimination (see Box 9.3). Use indifference curve analysis to explain why it may be diffi...
-
In comparing SAV among diseases and diagnoses, would more complicated diseases suggest greater or lesser variation?
-
Eightly insurance claim forms are inspected daily for 25 working days, and the number of forms with errors are recorded in the worksheet C16P7 in the OM6 Data Workbook. Construct a p-chart using the...
-
Refer to the data in QS 10-1. Based on financial considerations alone, should Helix accept this order at the special price? Explain.
-
Include activity coefficients from the Davies equation to find the pH and concentrations of species in the mixture of sodium tartrate, pyridinium chloride, and KOH in Section 12-1. Consider only...
-
(a) Using the ion-pair equilibrium constant from Appendix J, with activity coefficients = 1, find the concentrations of species in 0.025 M MgSO 4 . Hydrolysis of the cation and anion near neutral pH...
-
(a) Find the concentrations of species in saturated CaF 2 as a function of pH by using Reactions 12-32 through 12-36 and adding the following reaction: Do not include activity coefficients. Produce a...
-
The blue samurai, a japanese restraurant, has an asset turnover of 3.5 the total assets were 95,000 what are net sales for the blue samurai?
-
Gatekeeper Manufacturing reported 50,000 physical units that were 100% complete for direct materials during the period. In addition, the 50,000 physical units were 100% for conversion costs. In terms...
-
What red flag was overlooked on the Montague Fellowship Expense Report?

Study smarter with the SolutionInn App


