Question: Succinic acid dissociates in two steps: K1 H,H,C ,, + H* %3| || OCCH,CH,CO + H* , 3 2.3 10-6 HOCCH,CH,CO Calculate Kp1 and Kp2
Succinic acid dissociates in two steps:
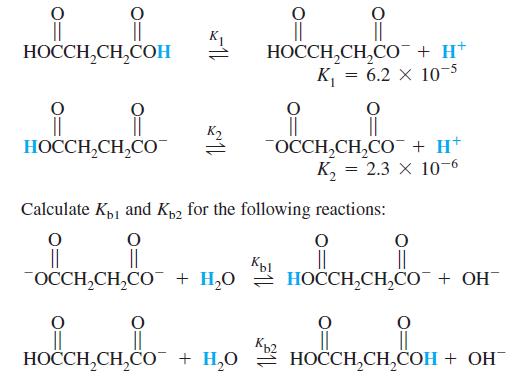
K1 H,H,C ,, + H* %3| || OCCH,CH,CO + H* , 3 2.3 10-6 HOCCH,CH,CO Calculate Kp1 and Kp2 for the following reactions: || OCCH,CH,CO + H,0 = HOCCH,CH,CO + OH Kp2 HOCCH,CH,CO + H,0 2 HOCCH,CH,COH + OH
Step by Step Solution
3.48 Rating (171 Votes )
There are 3 Steps involved in it
K1 1 HOOC CHzz COOH HOOC CHzz COOH K 62 x 105 On reversing the reaction K1 HOOC CH22 COOH HOOC C... View full answer

Get step-by-step solutions from verified subject matter experts


