Indicator error. Consider the titration in Figure 10-2 in which the equivalence-point pH in Table 10-2 is
Question:
Indicator error. Consider the titration in Figure 10-2 in which the equivalence-point pH in Table 10-2 is 9.25 at a volume of 10.00 mL.
(a) Suppose you used the yellow-to-blue transition of thymol blue indicator to find the end point. According to Table 10-3, the last trace of green disappears near pH 9.6. What volume of base is required to reach pH 9.6? The difference between this volume and 10 mL is the indicator error.
(b) If you used cresol red, with a color change at pH 8.8, what would be the indicator error?
Figure 10-2
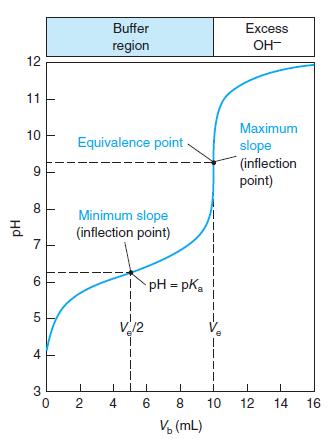
Table 10-2
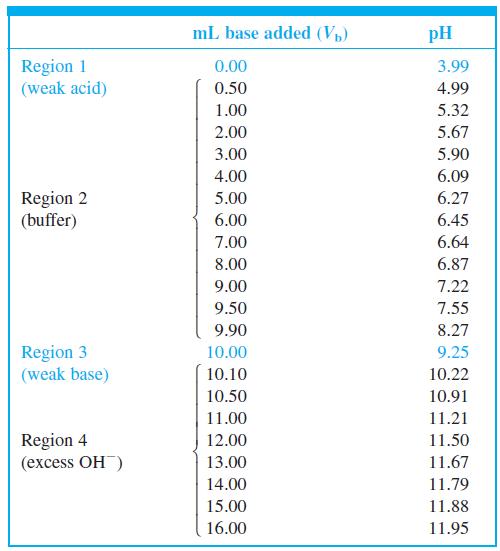
Buffer Excess region OH- 12 11 Maximum 10 Equivalence point slope (inflection point) Minimum slope (inflection point) 6. pH = pK, Ve/2 4 4 6 8. 10 12 14 16 V, (mL) 2. 7,
Step by Step Answer:
a The volume of base required to reach pH 96 can be calculated using the HendersonHasselbalch equati...View the full answer



Related Video
A substance that alters color in solution over a constrained range of pH values is known as a pH indicator or acid-base indicator. The indicator chemical just needs to alter color slightly in order to be noticed. Indicators work on the basis that they react with water to produce the hydrogen cation H+ or hydronium ion H3O+. The indicator molecule\\\'s color changes as a result of the reaction. Since indicators have distinct color ranges for color change, they can occasionally be combined to provide color changes over a wider pH range.
Students also viewed these Engineering questions
-
Consider the titration of 25.0 mL of 0.010 0 M Sn 2+ by 0.050 0 M Tl 3+ in 1 M HCl, using Pt and saturated calomel electrodes to find the end point. (a) Write a balanced titration reaction. (b) Write...
-
Consider the titration in Figure 10-2, for which the pH at the equivalence point is calculated to be 9.25. If thymol blue is used as an indicator, what color will be observed through most of the...
-
Consider the titration of 100.0 mL of 0.010 0 M Ce 4+ in 1 M HClO 4 by 0.040 0 M Cu+ to give Ce 3+ and Cu 2+ , using Pt and saturated Ag | AgCl electrodes to find the end point. (a) Write a balanced...
-
Suppose a wagon moves due east at 10.1 m/s while a skateboard heads pi 3 radians south of east at 12 m/s. What are the x- and y- components of the velocity of the wagon relative to the skateboard?
-
Does the research question stay the same throughout the research process? Why or why not?
-
If an increase in the current account deficit finances an increase in domestic investment, what implications does this have for the future performance of the economy?
-
You currently owe $\$ 18,000$ on a car loan at $9.5 \%$ interest. If you make monthly payments of $\$ 576.59$ per month, how long will it take you to fully repay the loan?
-
The following table shows how average share prices jump (in percentage) after the announcement that the stocks will be cross-listed (see Miller, 2000). The price response should be interpreted as...
-
Consider the recorded transactions below. Transaction Account Name Debit Credit 1. Accounts Receivable 7,400 Service Revenue 7,400 2. Supplies 1,800 Accounts Payable 1,800 3. Cash 9,200 Accounts...
-
In Parts I and II of this case, you performed preliminary analytical procedures and assessed acceptable audit risk and inherent risk for Pinnacle Manufacturing. Your team has been assigned the...
-
Finding the end point from pH measurements. Here are data points around the second apparent end point in Figure 10-5: (a) Prepare a spreadsheet or table analogous to Figure 10-6, showing the first...
-
Spectrophotometry with indicators. Acid-base indicators are themselves acids or bases. Consider an indicator, HIn, which dissociates according to the equation The molar absorptivity, , is 2 080 M -1...
-
A variable is normally distributed with mean 6 and standard deviation 2. Find the percentage of all possible values of the variable that a. Lie between 1 and 7. b. Exceed 5. c. Are less than 4.
-
a. The statutory code of your state probably has a number of different statutes that deal with children. Use its indexes to help you find as many of these statutes as you can (up to a maximum of...
-
Suppose that a statute in a state provides as follows: 10 No marriage shall be invalid on account of want of authority in any person solemnizing the same if consummated with the full belief on the...
-
Marcus begins trading on 1 January 2020 and has the following results: (a) Compute his trading income (before loss relief) for 2019-20 to 2021-22. (b) Identify the claims that could be made in...
-
Prepare a flowchart of the procedural steps that are necessary for a judicial separation in your state.
-
In what way(s) can an illegitimate child be legitimated in your state?
-
Vallarta Company recently reported notes payable and accrued payrolls and benefits as follows: Current liabilities (partial): ....................December 31, 2017... 2016 (in millions of dollars)...
-
Consider the advantages and disadvantages of extending property rights so that everyone would have the right to prevent people imposing any costs on them whatsoever (or charging them to do so).
-
Would tris(2,2 -bipyridine)iron be a useful indicator for the titration of Sn 2+ with Mn(EDTA) - ? (Hint: The potential at the equivalence point must be between the potentials for each redox couple.)
-
Explain what we mean by preoxidation and prereduction. Why is it important to be able to destroy the reagents used for these purposes?
-
Write balanced reactions for the destruction of S 2 O 2 -8 , Ag 2+, and H 2 O 2 by boiling.
-
This number is the "federal adjusted gross income" according to the line title. What do we call this process of starting our state return with a number from the federal return?
-
What is the main reason for a franchisee to have a contingency fund?
-
Kalan purchased four new tires at Walmart for $1,000 using there "no payments for 12 months plan." About a year later, before Kalan had made any payments, the tires were not performing as well as...

Study smarter with the SolutionInn App


