Use the systematic treatment of equilibrium to find the concentrations of the major species in a saturated
Question:
Use the systematic treatment of equilibrium to find the concentrations of the major species in a saturated aqueous solution of LiF. Consider these reactions:
![LiF(s) = Li* +F Ksp = [Li*]YLi+[F¯]YF Kjon pair 0.001 7 = LiF(aq)YLIF(aq) = 0.002 9 %3D LiF(s) = LiF(aq)](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1591/5/4/6/4645edd12603e2d41591546462162.jpg)
(a) Initially, set the ionic strength to 0 and solve for all concentrations. Then compute the ionic strength and activity coefficients and find new concentrations. Use several iterations to home in on the correct solution.
(b) In the systematic treatment, you will find that the calculation simplifies to 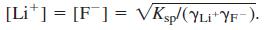 Set up the following spreadsheet, in which ionic strength in cell B4 is initially given the value 0. Activity coefficients in cells B6 and B8 are from the extended Debye-Hückel equation. Cell B10 computes
Set up the following spreadsheet, in which ionic strength in cell B4 is initially given the value 0. Activity coefficients in cells B6 and B8 are from the extended Debye-Hückel equation. Cell B10 computes 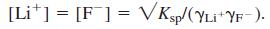 With 0 in cell B4, your spreadsheet should compute 1 in cells B6 and B8 and [Li+] = [F-] = 0.041 23 M in cell B10.
With 0 in cell B4, your spreadsheet should compute 1 in cells B6 and B8 and [Li+] = [F-] = 0.041 23 M in cell B10.
Step by Step Answer:





