Heterogeneous equilibria and calcite solubility. If river water in Box 7-2 is saturated with calcite (CaCO 3
Question:
Heterogeneous equilibria and calcite solubility. If river water in Box 7-2 is saturated with calcite (CaCO3), [Ca2+] is governed by the following equilibria:
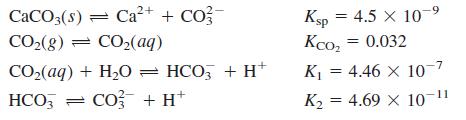
(a) From these reactions, find the equilibrium constant for the reaction
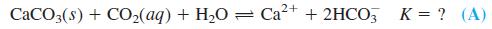
(b) The mass balance for Reaction A is [HC3-] = 2[Ca2+]. Find [Ca2+] (in mol/L and in mg/L) in equilibrium with atmospheric CO2 if PCO2 = 3.8 × 10-4bar. Locate this point on the line in Box 7-2.
(c) The concentration of Ca2+ in the Don River is 80 mg/L. What effective PCO2 is in equilibrium with this much Ca2+? How can the river have this much CO2?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





