Write the charge and mass balances for dissolving CaF 2 in water if the reactions are CaF,(8)
Question:
Write the charge and mass balances for dissolving CaF2 in water if the reactions are
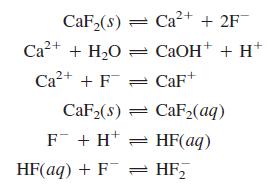
Transcribed Image Text:
CaF,(8) = Ca2+ + 2F Ca?+ + H,O = CAOH+ + H* Ca2+ + F = CaF+ CaF2(s) = CaF2(aq) F + H* = HF(aq) HF(aq) + F = HF,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
The charge for dissolving CaF 2 in ...View the full answer

Answered By

DURGAM SRAVANA KUMAR
I am Formerly mechanical engineering, I am worked as a tutor for college students in Graduation. I learn new things from internet and update my self.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Write charge and mass balances for aqueous Ca 3 (PO 4 ) 2 if the species are Ca 2+ , CaOH + , CaPO - 4 , PO 3- 4 , HPO 2 4 - , H 2 PO - 4 , and H 3 PO 4 .
-
State the meaning of the charge and mass balance equations.
-
(a) Write the charge and mass balances for a solution made by dissolving MgBr2 to give Mg2+, Br-, MgBr+, and MgOH+. (b) Modify the mass balance if the solution was made by dissolving 0.2 mol MgBr2 in...
-
Return to Better Mousetraps in Problem 18. Suppose the firm can cut its requirements for working capital in half by using better inventory control systems. By how much will this increase project NPV?...
-
A taxpayer is a partner in an accounting firm. He has always enjoyed auto racing as a hobby. Now that his children are grown, he has decided to devote more time to auto racing. He recently purchased...
-
A 10-kg stone is dropped into a pool of water from a height of 100 m. How much energy in joules does the stone have when it strikes the water? If all this energy goes into heat and if the pool...
-
Describe the significance of constraints and how they relate to business rules.
-
a.In the Maine Megabucks game, you win the jackpot by selecting five different whole numbers from 1 through 41 and getting the same five numbers (in any order) that are later drawn. What is the...
-
Question 22 Daffodil Corporation had the following information this year: Account Sales Gain on Sale of Truck Sales Discounts COGS Expenses Loss on Flood Damage Dividends Declared Unearned Revenue...
-
One way to think about wages for different jobs is to see it as another application of the law of one price. We came across this law when we discussed speculation in Chapter 7, and it came up again...
-
(a) Write the mass balance for CaCl 2 in water if the species are Ca 2+ and Cl - . (b) Write the mass balance if the species are Ca 2+ , Cl - , CaCl - , and CaOH + . (c) Write the charge balance for...
-
Using activities, find the concentrations of the major species in 0.10 M NaClO 4 saturated with Mn(OH) 2 . Take the ionic strength to be 0.10 M and suppose that the ion size of MnOH + is the same as...
-
For the office building in the preceding table, compute the following for 2007: a. Monthly natural gas consumption based on Btu/ft 2 of floor area. b. Annual natural gas consumption based on Btu/ft 2...
-
What is the slope of the isoquant described by the data in Table 2.5 when evaluated from a labor input of 7 to 8? Q Table 2.5 An Isoquant Schedule K L 10 10 10 10 10 10 11 10 10 1578022 100,000.00...
-
In a new business during the year ended 31 December 2013 the following debts are found to be bad, and are written-off on the dates shown: On 31 December 2013 the schedule of remaining accounts...
-
Enter up the relevant accounts in the purchases and general ledgers from the columnar purchases day book you completed for Question 20.4. Question 20.4 A Enter up a columnar purchases day book with...
-
Two large manufacturers make similar products but have different branding strategies: one houses all of its products under the parent brand, but the other has a collection of different brands that...
-
If someone reports that the mean weight for fourth-grade boys is 80 pounds and for fourth-grade girls is 78 pounds, what must you know to test hypotheses using the difference of means?
-
A firm is evaluating the alternative of manufacturing a part that is currently being outsourced from a supplier. The relevant information is provided below: For in-house manufacturing: Annual fixed...
-
Should U.S. antidumping laws be stated in terms of average total costs or average variable costs?
-
Sulfide ion was determined by indirect titration with EDTA. To a solution containing 25.00 mL of 0.04332 M Cu(ClO4)2 plus 15 mL of 1M acetate buffer (pH 4.5) were added 25.00 mL of unknown sulfide...
-
Cesium ion does not form a strong EDTA complex, but it can be analyzed by adding a known excess of NaBiI4 in cold concentrated acetic acid containing excess NaI. Solid Cs3Bi2I9 is precipitated,...
-
The sulfur content of insoluble sulfides that do not readily dissolve in acid can be measured by oxidation with Br2 to SO2-4.25 Metal ions are then replaced with H+ by an ion exchange column, and...
-
What is Fibonacci heap? Explain CONSOLIDATE operation with suitable example for Fibonacci heap ?
-
Discuss the impact of global supply chain disruptions, exacerbated by events like the COVID-19 pandemic, on inventory management strategies and market resilience across various industries?
-
A pharmaceutical retailer decided to host a website for home delivery of medicines according to user orders. The web application is deployed on a single Amazon EC2 instances. within a few months, the...

Study smarter with the SolutionInn App


