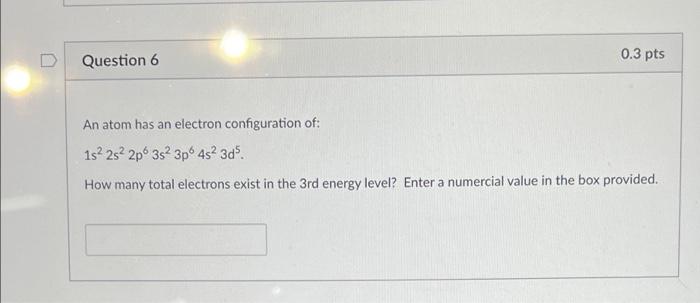
Question: 0.3 pts Question 6 An atom has an electron configuration of: 1s 2s 2p 3s 3p 452 3d5. How many total electrons exist in the

0.3 pts Question 6 An atom has an electron configuration of: 1s 2s 2p 3s 3p 452 3d5. How many total electrons exist in the 3rd energy level? Enter a numercial value in the box provided. Question 7 0.3 pts A student is trying to write the condensed electron configuration for iron (Fe). What Group 8A element should the student use as a starting point in this process? O [Ar] OK! (He) (Ne) 0 [X] O [R]
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


