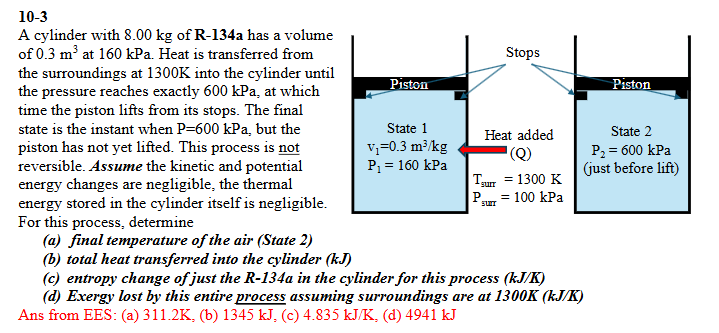
Question: ( 1 0 - 3 ) A cylinder with 8 . 0 0 kg of R - 1 3 4 a has a
A cylinder with kg of Ra has a volume of mathrm~m at kPa Heat is transferred from the surroundings at K into the cylinder until the pressure reaches exactly kPa at which time the piston lifts from its stops. The final state is the instant when mathrmPmathrmkPa but the piston has not yet lifted. This process is not reversible. Assume the kinetic and potential energy changes are negligible, the thermal energy stored in the cylinder itself is negligible. For this process, determine
a final temperature of the air State
b total heat transferred into the cylinder kJ
c entropy change of just the R a in the cylinder for this process mathbfk JmathrmK
d Exergy lost by this entire process assuming surroundings are at K kJK Ans from EES: a K b kJ cmathrm~kJmathrmKd kJ
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


