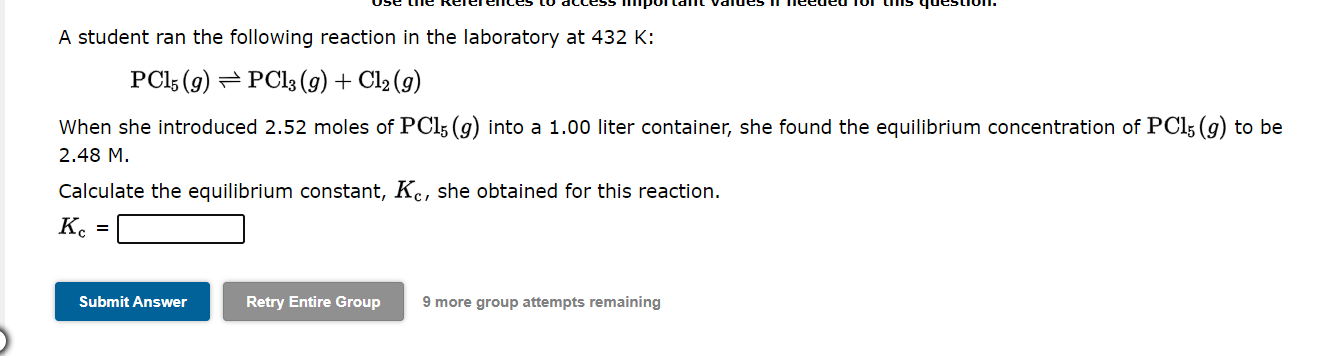
Question: 1. 2. 3. PLEASE ANSWER ALL THREE QUESTIONS AND ALL PARTS. A student ran the following reaction in the laboratory at 432K : PCl5(g)PCl3(g)+Cl2(g) When
1. 
2.
3. 
PLEASE ANSWER ALL THREE QUESTIONS AND ALL PARTS.
A student ran the following reaction in the laboratory at 432K : PCl5(g)PCl3(g)+Cl2(g) When she introduced 2.52 moles of PCl5(g) into a 1.00 liter container, she found the equilibrium concentration of PCl5(g) to be 2.48M. Calculate the equilibrium constant, Kc, she obtained for this reaction. Kc= 9 more group attempts remaining The equilibrium constant, Kc, for the following reaction is 1.54103 at 237K. 2NOBr(g)2NO(g)+Br2(g) When a sufficiently large sample of NOBr(g) is introduced into an evacuated vessel at 237K, the equilibrium concentration of Br2(g) is found to be 0.117M. Calculate the concentration of NOBr in the equilibrium mixture. [NOBr]=M 9 more group attempts remaining A student ran the following reaction in the laboratory at 737K : H2(g)+I2(g)2HI(g) When she introduced 0.189 moles of H2(g) and 0.213 moles of I2(g) into a 1.00 liter container, she found the equilibrium concentration of HI(g) to be 0.311M. Calculate the equilibrium constant, Kc, she obtained for this reaction. Kc=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


