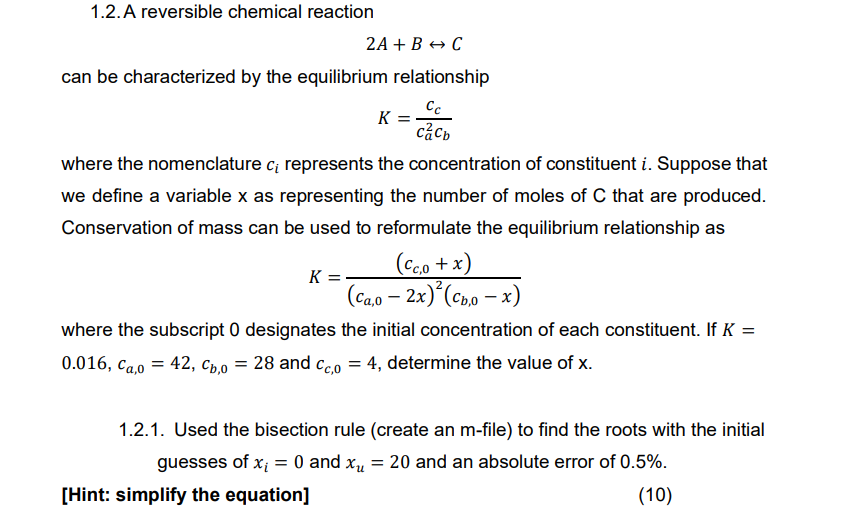
Question: 1 . 2 . A reversible chemical reaction 2 A + BharrC can be characterized by the equilibrium relationship K = c c c a
A reversible chemical reaction
BharrC
can be characterized by the equilibrium relationship
where the nomenclature represents the concentration of constituent Suppose that
we define a variable as representing the number of moles of that are produced.
Conservation of mass can be used to reformulate the equilibrium relationship as
where the subscript designates the initial concentration of each constituent. If
and determine the value of
Used the bisection rule create an m file to find the roots with the initial
guesses of and and an absolute error of
Hint: simplify the equation using matlab
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


