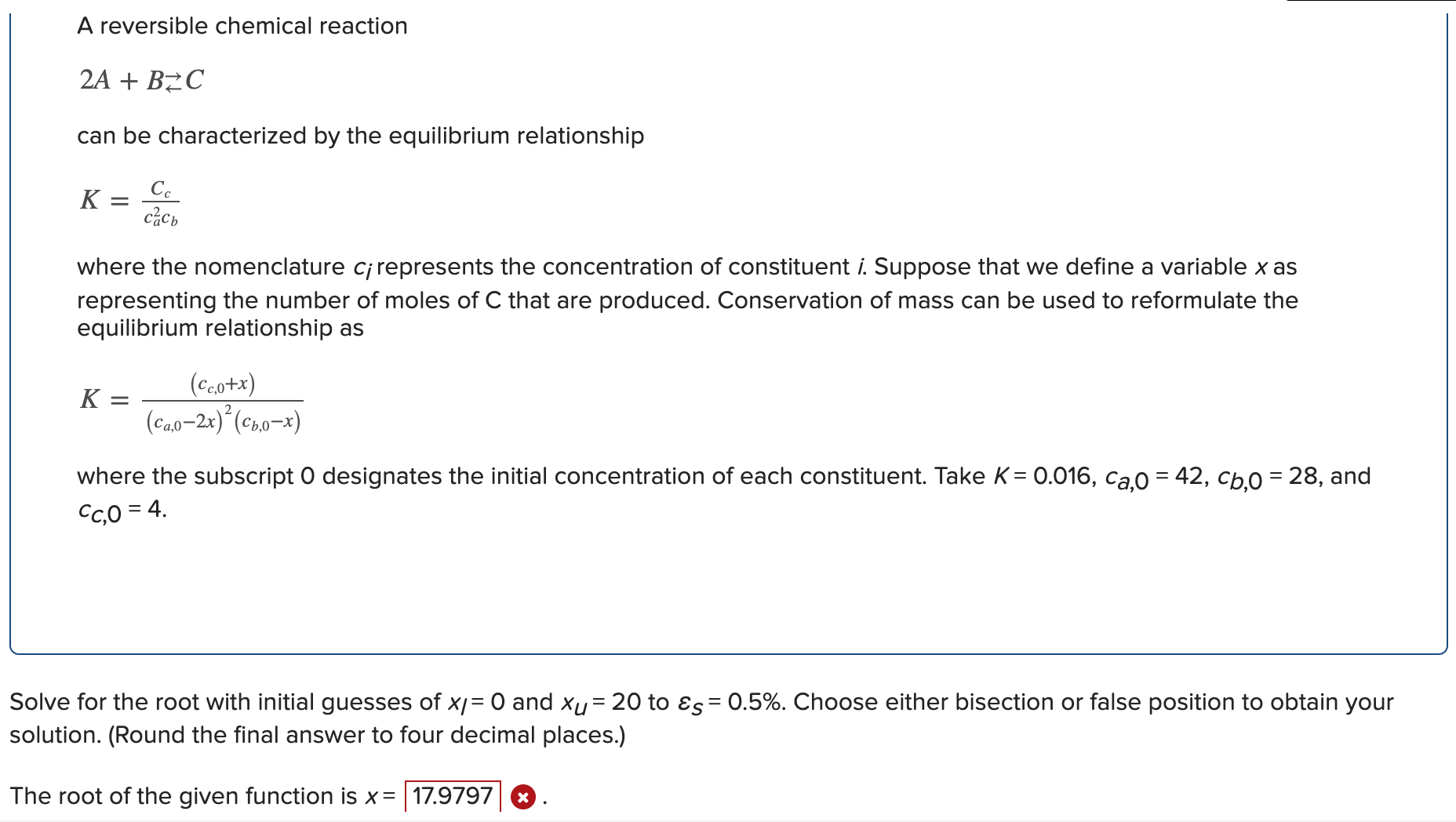
Question: Solve for the answer I got incorrect at the bottom A reversible chemical reaction 2A+BC can be characterized by the equilibrium relationship K=ca2cbCc where the
 Solve for the answer I got incorrect at the bottom
Solve for the answer I got incorrect at the bottom
A reversible chemical reaction 2A+BC can be characterized by the equilibrium relationship K=ca2cbCc where the nomenclature ci represents the concentration of constituent i. Suppose that we define a variable x as representing the number of moles of C that are produced. Conservation of mass can be used to reformulate the equilibrium relationship as K=(ca,02x)2(cb,0x)(cc,0+x) where the subscript 0 designates the initial concentration of each constituent. Take K=0.016,ca,0=42,cb,0=28, and cC,0=4 Solve for the root with initial guesses of xI=0 and xU=20 to S=0.5%. Choose either bisection or false position to obtain your olution. (Round the final answer to four decimal places.) he root of the given function is x=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


