Question: please solve it with clear steps using MATLAB Q4) A reversible chemical reaction: 2A+BC, can be characterized by the equilibrium relationship: K=cadcccc where the nomenclature
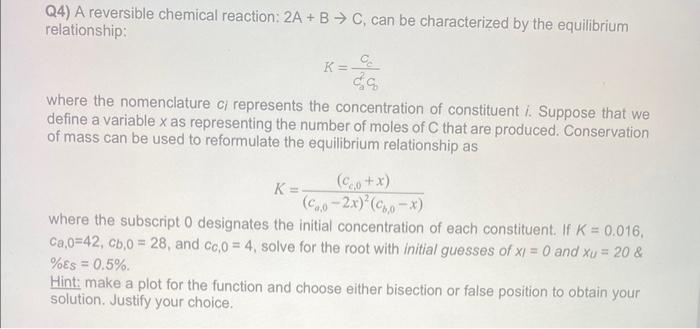
Q4) A reversible chemical reaction: 2A+BC, can be characterized by the equilibrium relationship: K=cadcccc where the nomenclature ci represents the concentration of constituent i. Suppose that we define a variable x as representing the number of moles of C that are produced. Conservation of mass can be used to reformulate the equilibrium relationship as K=(ca,02x)2(cb,0x)(cc,0+x) where the subscript 0 designates the initial concentration of each constituent. If K=0.016, ca,0=42,cb,0=28, and cc,0=4, solve for the root with initial guesses of xI=0 and xu=20 \& %s=0.5% Hint: make a plot for the function and choose either bisection or false position to obtain your solution. Justify your choice
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


