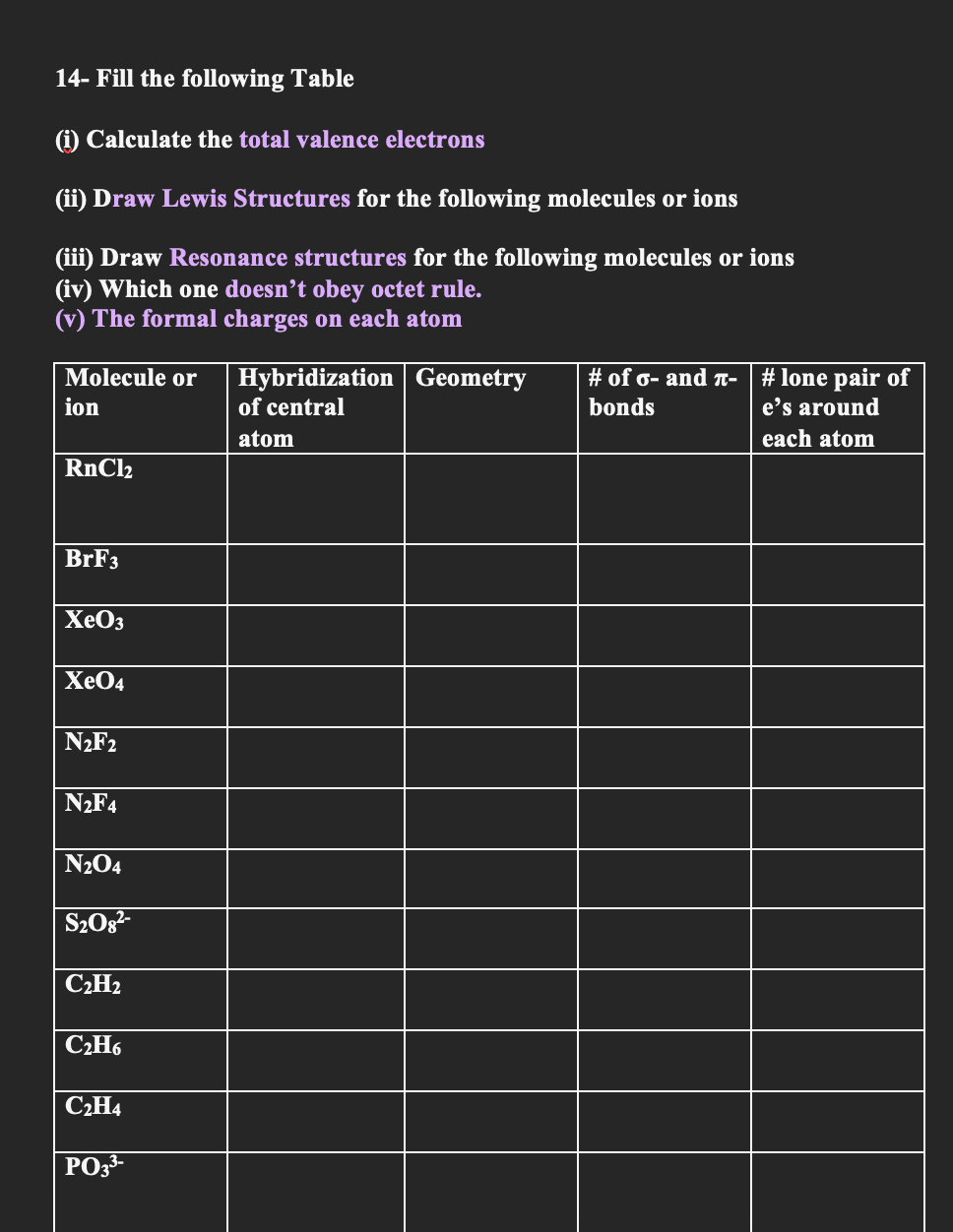
Question: 1 4 - Fill the following Table ( i ) Calculate the total valence electrons ( ii ) Draw Lewis Structures for the following molecules
Fill the following Table
i Calculate the total valence electrons
ii Draw Lewis Structures for the following molecules or ions
iii Draw Resonance structures for the following molecules or ions
iv Which one doesn't obey octet rule.
v The formal charges on each atom
tabletableMolecule oriontableHybridizationof centralatomGeometry,table# of and bondstable# lone pair ofes aroundeach atom
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


