Question: 1. (40 P) In a constant volume batch reactor, acetaldehyde decomposes to methane and carbon monoxide at an elevated temperature. During the reaction, the increase
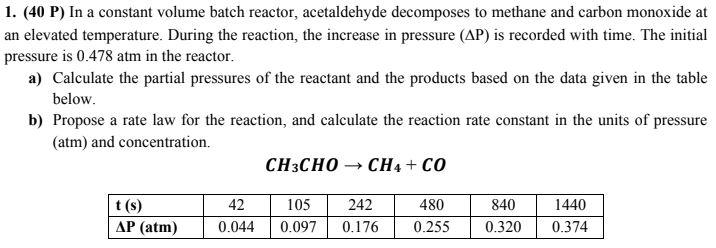
1. (40 P) In a constant volume batch reactor, acetaldehyde decomposes to methane and carbon monoxide at an elevated temperature. During the reaction, the increase in pressure (P) is recorded with time. The initial pressure is 0.478atm in the reactor. a) Calculate the partial pressures of the reactant and the products based on the data given in the table below. b) Propose a rate law for the reaction, and calculate the reaction rate constant in the units of pressure (atm) and concentration. CH3CHOCH4+CO
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


