Question: 1. (a) Explain how does an ion-exchange process work? (b) Equilibrium isotherm data for adsorption of glucose from an aqueous solution to activated alumina are
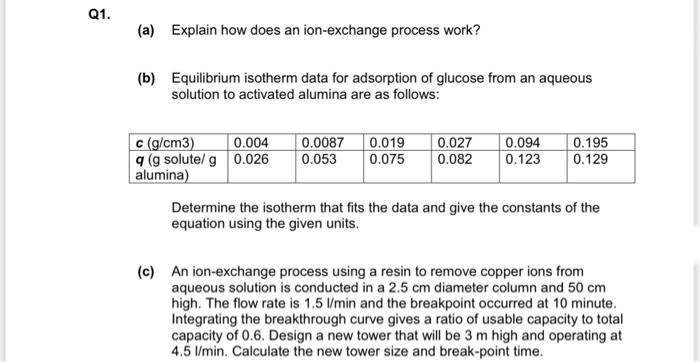
1. (a) Explain how does an ion-exchange process work? (b) Equilibrium isotherm data for adsorption of glucose from an aqueous solution to activated alumina are as follows: Determine the isotherm that fits the data and give the constants of the equation using the given units. (c) An ion-exchange process using a resin to remove copper ions from aqueous solution is conducted in a 2.5cm diameter column and 50cm high. The flow rate is 1.5l/min and the breakpoint occurred at 10 minute. Integrating the breakthrough curve gives a ratio of usable capacity to total capacity of 0.6 . Design a new tower that will be 3m high and operating at 4.5l/min. Calculate the new tower size and break-point time
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


